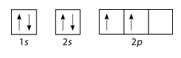5.18 电子配置
章节大纲
-
How big is a file?
::档案有多大?If you keep your papers in manila folders, you can pick up a folder and see how much it weighs. If you want to know how many different papers (articles, bank records, or whatever else you keep in a folder), you have to take everything out and count. A computer directory, on the other hand, tells you exactly how much you have in each file. We can get the same information on atoms. If we use an filling diagram, we have to count arrows. When we look at electron configuration data, we simply add up the numbers.
::如果您将您的文件保存在 manila 文件夹中, 您可以取一个文件夹, 并看它有多重。 如果您想知道有多少不同的文件( 文章、 银行记录, 或者您在文件夹中保存的其他文件) , 您必须把所有的文件都拿出来进行计算。 另一方面, 计算机目录可以告诉您每个文件中的确切数量 。 我们可以在原子上获得同样的信息 。 如果我们使用填充图, 我们就必须计算箭头。 当我们查看电子配置数据时, 我们只需将数字加在一起 。Electron Configurations
::电子配置Electron configuration notation eliminates the boxes and arrows of orbital filling diagrams. Each occupied sublevel designation is written followed by a superscript that is the number of electrons in that sublevel. For example, the hydrogen configuration is 1 s 1 , while the helium configuration is 1 s 2 . Multiple occupied sublevels are written one after another. The electron configuration of lithium is 1 s 2 2 s 1 . The sum of the superscripts in an electron configuration is equal to the number of electrons in that , which is in turn equal to its .
::电子配置标记消除了轨道填充图的框和箭头。 每个已占用的子级别指定后都写上一个上标, 即该子级别上电子的数量。 例如, 氢配置为 1s1, 配置为 1s2 ; 多位占用子级别是逐字写入的。 锂的电子配置为 1s22s1 。 电子配置中的上标数和电子配置中的电子数量相等, 而后者又等于电子数量 。Sample Problem: Orbital Filling Diagrams and Electron Configurations
::问题:轨道填充图和电子配置Draw the orbital filling diagram for carbon and write its electron configuration.
::绘制碳的轨道填充图并写入其电子配置。Step 1: List the known quantities and plan the problem.
::第1步:列出已知数量并规划问题。Known
::已知已知-
atomic number of carbon, Z = 6
::碳原子数,Z = 6
Use the order of fill diagram to draw an orbital filling diagram with a total of six electrons. Follow Hund’s rule . Write the electron configuration.
::使用填充图的顺序绘制轨道填充图,共包含六个电子。 遵循洪德规则。 写入电子配置 。Step 2: Construct Diagram.
::第2步:构建图表。Orbital filling diagram for carbon.
::碳轨道填充图。Electron configuration 1 s 2 2 s 2 2 p 2
::电子配置 1s22s22p2Step 3: Think about your result.
::步骤3:想想你的结果。Following the 2 s sublevel is the 2 p , and p sublevels always consist of three orbitals. All three orbitals need to be drawn even if one or more is unoccupied. According to Hund’s rule, the sixth enters the second of those p orbitals and with the same spin as the fifth electron.
::2级以下是 2 点,而 p 级以下总是由 3 个轨道组成。 所有 3 个 轨道 都需要 绘制 , 即使一个或一个以上没有被占用 。 根据 洪德 规则 , 第 6 个轨道进入第二 个轨道 , 与 第 5 个 电子 具有相同的旋转 。Second Period Elements
::第二期间 要件Periods refer to the horizontal rows of the periodic table . Looking at a periodic table you will see that the first period contains only the hydrogen and helium. This is because the first principal energy level consists of only the s sublevel and so only two electrons are required in order to fill the entire principal energy level. Each time a new principal energy level begins, as with the third element lithium, a new period is started on the periodic table. As one moves across the second period, electrons are successively added. With beryllium (Z = 4), the 2 s sublevel is complete and the 2 p sublevel begins with boron (Z = 5). Since there are three 2 p orbitals and each orbital holds two electrons, the 2 p sublevel is filled after six elements. The Table shows the electron configurations of the elements in the second period.
::指的是周期表的横向行。 查看周期表时, 可以看到第一个周期只包含氢和。 这是因为第一个主要能源级别仅包含子级, 所以要填满整个主要能源级别只需要两个电子。 每次新的主要能源级别开始, 与第三个元素锂一样, 周期表上开始一个新的时期。 当一个周期跨第二个周期移动时, 电子会连续添加。 使用( Z= 4) , 2个子级别是完整的, 2p 子级别以( Z= 5) 开始, 因为有3个 2p 轨道, 每个轨道有2 电子, 2p 子级别在6个元素之后填充。 表格显示了第二个周期各元素的电子配置 。Electron Configurations of Second-Period Elements Element Name Symbol Atomic Number Electron Configuration Lithium Li 3 1 s 2 2 s 1 Beryllium Be 4 1 s 2 2 s 2 Boron B 5 1 s 2 2 s 2 2 p 1 Carbon C 6 1 s 2 2 s 2 2 p 2 Nitrogen N 7 1 s 2 2 s 2 2 p 3 Oxygen O 8 1 s 2 2 s 2 2 p 4 Fluorine F 9 1 s 2 2 s 2 2 p 5 Neon Ne 10 1 s 2 2 s 2 2 p 6 Further Reading
::继续阅读Hund's Rule and Filling Diagrams
::百人规则和填充图Summary
::摘要-
Electron configuration notation simplifies the indication of where electrons are located in a specific atom.
::电子配置标记简化了表明电子在特定原子中的位置。 -
Superscripts are used to indicate the number of electrons in a given sublevel.
::上标用于表示一个子级别中的电子数量。
Review
::回顾-
What does electron configuration notation eliminate?
::电子配置符号消除了什么? -
How do we know how many electrons are in each sublevel?
::我们怎么知道每个子层有多少电子? -
An atom has the electron configuration of 1
s
2
2
s
2
2
p
5
. How many electrons are in that atom?
::原子的电子配置为 1s22s22p5. 原子中有多少电子? -
Which element has the electron configuration of 1
s
2
2
s
2
2
p
6
3
s
2
?
::哪个元素的电子配置为 1s22s22p632 ?
-
atomic number of carbon, Z = 6