6.12 卤素
章节大纲
-
How do you study a gas that does not exist as such in nature?
::您如何研究一种性质上不存在的气体?It’s not as easy as you think. Fluorine is so reactive that we cannot find it free in nature. None of the halogens exist free in nature (unlike some of the such as gold and silver) because they are very reactive. The video below shows how violently elemental fluorine reacts with other materials.
::这并不像你们想象的那么容易。 氟的活性太强,以至于我们无法在自然界找到自由。 卤素中没有一个(不像金银等金银等)是自由的,因为它们是非常被动的。 下面的视频显示了元素氟与其他材料的强烈反应。Halogens
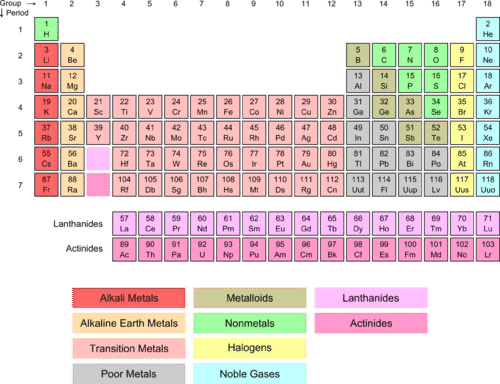
::卤素Some are much more reactive than others. The Group I (red) and Group II (tan) elements can easily lose electrons during a reaction. Elements of other groups are much more likely to accept electrons as they react.
::第一组(红色)和第二组(丹)元素在反应中很容易失去电子,其他组的元素在反应中接受电子的可能性大得多。The elements of Group VIIA (new Group 17 - fluorine, chlorine, bromine, iodine, and astatine) are called the halogens (yellow column). The term “halogen” means “salt-former” because these elements will readily react with alkali metal and to form halide salts . The halogens all have the general electron configuration ns 2 np 5 , giving them seven . They are one short of having the full outer s and p sublevel, which makes them very reactive.
::第七A组(新的第17组 -- -- 氟、氯、溴、碘和阿 Statine)的成分称为卤素(黄柱), " 卤素 " 是指 " 盐前 " ,因为这些元素将很容易与碱性金属发生反应并形成卤化盐,卤素都具有一般电子配置ns2np5, 给它们7个。它们缺少全部外表和子层,因此非常反应性。Physical Properties of Halogens
::卤素的物理特性As elements, chlorine and fluorine are at room temperature , bromine is a dark orange , and iodine is a dark purple-gray solid. Astatine is so rare that its properties are mostly unknown. In the picture below we see chlorine gas on the left (green), bromine solid and vapor in the middle (orange), and solid iodine (grey) on the right. Fluorine is not shown in the picture below because it is too corrosive and will destroy the glass container.
::由于元素、氯和氟处于室温,溴是一种深橙,碘是一种深紫色固体,而碘则是一种深紫色的紫色固体。阿 Statine非常罕见,其特性大多不为人所知。在下图中,我们看到左边的氯气(绿色)、中间的溴固体和蒸气(橙色)以及右边的固态碘(灰色),下面的图中没有显示氟,因为它腐蚀性过强,会摧毁玻璃容器。None of these elements are found free in nature because of their reactivity. Fluorine is found in combination with in several minerals. Chlorine is found in table salt, in the oceans (which are about 2% chloride by weight) and in lakes such as the Great Salt Lake in Utah. Small amounts of bromide and iodide salts can be found in the oceans and in brine wells in several states.
::这些元素中没有一个由于其反应性而具有自由性质,氟与若干矿物结合被发现,氯在桌盐、海洋(按重量氯化约2%)和犹他州大盐湖等湖泊中被发现,在几个州,在海洋和盐井中可以找到少量的溴和碘化盐。Watch the following two video experiments of p block elements:
::观看以下两个P区块元素的视频实验:This first video is of bromine reacting with aluminum.
::第一部影片是 溴与铝反应。This second video discusses the properties of halogens and shows a few more reactions they can participate in.
::第二段影片讨论卤素的特性,Summary
::摘要-
The halogens all have seven electrons in their outer shells.
::卤素外壳里都有七个电子 -
The electron configuration in the outer shell is
ns
2
np
5
.
::外壳的电子配置为 ns2np5。 -
As the atomic number increases, the reactivity of the halogens decreases.
::随着原子数的增加,卤素的回弹性下降。 -
Fluorine and chlorine exist as gases at room temperature, while bromine is a liquid, and iodine is a solid.
::氟和氯作为气体存在于室内温度下,而溴是一种液体,碘是一种固体。
Review
::回顾-
Pick two elements that are halogens. For each, write the name, chemical symbol, and atomic weight.
::选择两种卤素元素。 对于每一种元素, 写上名称、 化学符号和原子重量 。 -
What does the term “halogen” mean?
::“卤素”一词是什么意思? -
What is the outer shell electron configuration for the halogens?
::卤素的外壳电子配置是什么? -
What is the physical state of each halogen at room temperature?
::室温下每种卤素的物理状态如何? -
Where are the halogens found?
::卤素在哪里被发现?
-
The halogens all have seven electrons in their outer shells.