6.21 定期趋势:电子密度
章节大纲
-
Is it easy or hard for you to make new friends?
::你交新朋友是容易还是难?Have you ever noticed how some people attract others to them? Whether it is their personality, attractiveness, or athletic skills – something pulls people toward them, while others have a smaller group of friends and acquaintances. Atoms do the same thing. One may pull electrons strongly to it, while a second type of atom has much less “pulling power.”
::你难道没有注意到有些人是如何吸引他人的吗?不管是他们的个性、吸引力还是运动技能 — — 某些东西将人们吸引到他们身边,而另一些人则有较少的朋友和熟人。 原子也做同样的事情。 人们可能会将电子引向它,而另一种原子则少得多的“拉动能力 ” 。Electronegativity
::电能of both atoms are always involved when those two atoms come together to form a . Chemical bonds are the basis for how combine with one another to form compounds. When these chemical bonds form, atoms of some elements have a greater ability to attract the valence electrons involved in the bond than other elements.
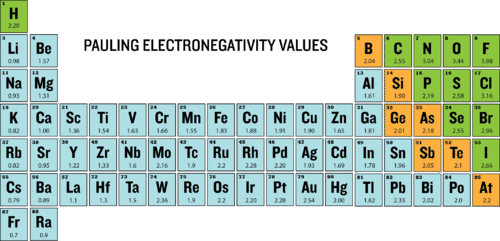
::当这两个原子聚集在一起形成一个原子时,这两个原子总是涉及两个原子。化学债券是如何相互结合形成化合物的基础。当这些化学债券形成时,某些元素的原子比其他元素更有能力吸引债券中涉及的价值电子。Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a . Electronegativity differs from electron affinity because electron affinity is the actual energy released when an atom gains an . Electronegativity is not measured in energy units, but is rather a relative scale. All elements are compared to one another, with the most electronegative element, fluorine, being assigned an electronegativity value of 3.98. Fluorine attracts electrons better than any other element. The table below shows the electronegativity values for the elements.
::当原子是原子的一部分时,电子感能是原子吸引电子能力的一种尺度。 电子感能与电子亲近性不同,因为电子亲近性是原子获得原子时释放的实际能量。 电子感能不是用能量单位来衡量的,而是相对的尺度。 所有的元素都是相互比较的, 最电子性元素为氟, 其电子性值为3. 98。 氟素吸引电子的优于任何其他元素。 下表显示了元素的电子性值。The electronegativity scale was developed by Nobel Prize winning American chemist Linus Pauling. The largest electronegativity (3.98) is assigned to fluorine and all other electronegativities measurements are on a relative scale. Since have few valence electrons, they tend to increase their stability by losing electrons to become . Consequently, the electronegativities of metals are generally low. have more valence electrons and increase their stability by gaining electrons to become . The electronegativities of nonmetals are generally high.
::由于价值电子很少,它们往往会因失去电子而增加稳定性,因此,金属的电子密度一般较低。 金属的值电子值比较高,通过获得电子来增加稳定性。 非金属的电子密度一般很高。Trends
::趋势趋势趋势趋势趋势趋势趋势趋势趋势趋势Electronegativities generally increase from left to right across a period. This is due to an increase in nuclear charge. Alkali metals have the lowest electronegativities, while have the highest. Because most do not form compounds, they do not have electronegativities. Note that there is little variation among the . Electronegativities generally decrease from top to bottom within a group due to the larger atomic size.
::电能在一段时间内通常会从左向右增长。 这是由于核电量的增加。 阿尔卡利金属是最低电子电能, 而其含量最高。 由于大多数金属没有形成化合物, 它们没有电子电能。 请注意, 电能在组内从上到下一般会因原子大小较大而下降。Of the main group elements, fluorine has the highest electronegativity (EN = 4.0) and cesium the lowest (EN = 0.79). This indicates that fluorine has a high tendency to gain electrons from other elements with lower electronegativities. We can use these values to predict what happens when certain elements combine.
::在主要组元素中,氟具有最高的电子密度(EN = 4.0)和最低的(EN = 0.79),这表明氟具有从电子密度较低的其他元素获取电子的高度倾向。我们可以使用这些值来预测某些元素结合时会发生什么。When the difference between electronegativities is greater than ~1.7, then a complete exchange of electrons occurs. Typically this exchange is between a metal and a nonmetal. For instance, sodium and chlorine will typically combine to form a new compound and each becomes isoelectronic with its nearest noble . When we compare the EN values, we see that the electronegativity for Na is 0.93 and the value for Cl is 3.2. The absolute difference between ENs is |0.93 - 3.2| = 2.27. This value is greater than 1.7, and therefore indicates a complete electron exchange occurs.
::当电子能量的差值大于~1.7时,就会发生电子的完全交换。这种交换通常是在金属和非金属之间。例如,钠和氯通常会合并成一个新的化合物,每种化合物都会变成与最接近的贵重的等电子化。当我们比较EN值时,我们看到Na的电子密度为0.93,而Cl的值为3.2。ENs之间的绝对差值是.93 - 3.2=2.27。这个值大于1.7,因此表示发生了完全的电子交换。Summary
::摘要-
Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound.
::当原子是化合物的一部分时,电能是原子吸引电子的能力的衡量标准。 -
Electronegativity values generally increase from left to right across the periodic table.
::周期表中的电效应值通常会从左向右增长。 -
Electronegativities generally decrease from top to bottom of a group.
::电能通常会从一个组的上到下下降。 -
The highest electronegativity value is for fluorine.
::最高的电子电子密度值是氟。
Review
::回顾-
Define “electronegativity.”
::“电子能力”的定义是“电子能力”。 -
How does electronegativity differ from electron affinity?
::电子亲近性与电子亲近性有何不同? -
Why are the electronegativity values of metals generally low?
::为什么金属的电子浓缩值普遍较低? -
Describe the trend in electronegativities across the periodic table.
::说明各周期表中电子指数的趋势。 -
Describe the trends in electronegativities in a group of the periodic table.
::在一组定期表格中描述电子能源的趋势。
-
Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound.