7.3 说明
章节大纲
-
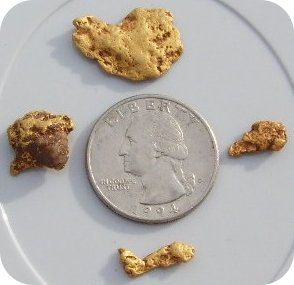
Have you ever gone digging for gold?
::你挖过金子吗?When the prospectors during the California Gold Rush (1848-1855) searched for gold nuggets in the earth, they were able to find these nuggets because gold is an unreactive material that exists in its elemental state in many places. Not everyone was fortunate enough to find large nuggets such as those shown above, but a number of these miners did become very wealthy (of course, a large number of others went back home broke).
::当加州金矿开采期间(1848-1855年)探矿者在地球上寻找金块时,他们找到了这些金块,因为黄金是其元素状态中存在于许多地方的无反应物质。 并非每个人都幸运地找到了上面提到的大型金块,但其中一些矿工确实变得非常富有(当然,还有很多人破产回家 ) 。Many of the we know about do not exist in their native form. They are so reactive that they are found only in compounds. These non-elemental forms are known as . Their properties are very different from those of the elements they come from. The term comes from a Greek word meaning “move” and was first coined by Michael Faraday, who studied the movement of materials in an electrical field.
::我们所知道的许多事物并不以其本源形式存在。 它们非常被动, 只在化合物中被发现。 这些非神经性的形式被称为 。 它们的性质与它们来自的元素非常不同。 这个词来自希腊语中的“ 移动 ” 一词, 最初是由迈克尔· 法拉第发明的, 他研究的是电子领域材料的移动。Cations
::刻记Sodium loses an electron to become a cation.
::钠会失去一个电子 变成一个电极Some elements lose one or more electrons in forming ions. These ions are known as “ cations ” because they are positively charged and migrate toward the negative electrode ( cathode ) in an electrical field. Looking at the periodic table below, we know that the group 1 elements are all characterized by having one s in the outer orbit and group 2 elements have two s electrons in the outer orbit. These electrons are loosely attached to the and can easily be removed, leaving more in the atom that there are electrons, so the resulting ion has a positive charge. Cations can also be formed from electron loss to many of the .
::有些元素在形成离子时会失去一个或多个电子。 这些离子被称为“ 电离子 ” , 因为它们是正电荷, 并迁移到电场中的负电极( 阴极) 。 从下表的周期表看, 我们知道第1组元素的特征都是在外轨道上有一秒, 第2组元素在外轨道上有两个电子。 这些电子松散地附着在外轨道上,并且很容易被移走, 在原子中留下更多的电子, 因而产生的离子具有正电荷。 电离子也可以从许多电子损失中形成。Periodic table of elements, notated with group numbers.
::元素周期表,用组号标明。The cations are designated by the symbol for the parent element and a plus charge as a superscript after the element symbol - the potassium cation would be indicated as K + . Note that the charge is placed after the symbol and not before it. The potassium ion is monovalent , meaning that it has lost one electron and has a 1 + charge. The symbol for the magnesium cation would be Mg 2+ or Mg ++ to indicate that it has lost two electrons and has a 2 + charge, so the magnesium cation would be referred to as a divalent cation.
::电荷由母元素的符号指定,外加电荷作为元素符号之后的上标 -- -- 钾加注将表示为K+。请注意,电荷放在符号之后,而不是放在符号之前。钾离子是单价的,这意味着它损失了一个电子,并且有一个1+电荷。镁加注的符号是Mg2+或Mg++,以表明它损失了两个电子,并且有2+电荷,因此镁加注将被称为二价电。The cations are simply named as the parent element. The sodium cation is still called “sodium.” Often, the charge would be attached for clarity, so the sodium cation might be referred to as “sodium one plus.”
::氯化钠仅被命名为母体元素。 氯化钠仍被称为“钠 ” 。 通常情况下,电荷会被附加为清晰度,因此氯化钠可以被称为“一加钠 ” 。Applications of Cations
::文化应用Cations play important roles in our daily lives. Sodium, potassium, and magnesium ions are essential for such processes as blood pressure regulation and muscle contraction. Calcium ion is an important part of bone structure. Sodium ions can used in water softeners to remove other harmful elements. We put sodium chloride (table salt) on our food and use it as a preservative.
::、钾和镁离子对于血压调节和肌肉收缩等过程至关重要。 钙离子是骨骼结构的重要组成部分。 钠离子可用于水柔软器中去掉其他有害元素。 我们把氯化钠(可食盐)放在食物上,并用作防腐剂。Summary
::摘要-
Cations are formed by the loss of one or two electrons from an element.
::元件损失一或两个电子构成电离层。 -
Groups 1 and 2 elements form cations.
::第1组和第2组要素构成电弧。 -
Cations are named according to the parent element.
::配方按母体元素命名。 -
Cation charges are indicated with a superscript following the chemical symbol.
::装药在化学符号后面用上标符号表示。
Review
::回顾-
What is a cation?
::什么是圆珠? -
Write the symbol for the barium cation.
::写作酸的符号。 -
Write the symbol for the cesium cation.
::刻写酸的符号。 -
List three ways cations are useful.
::列举三种方式是有用的。
-
Cations are formed by the loss of one or two electrons from an element.