Lewis Electron-Dot 结构
章节大纲
-
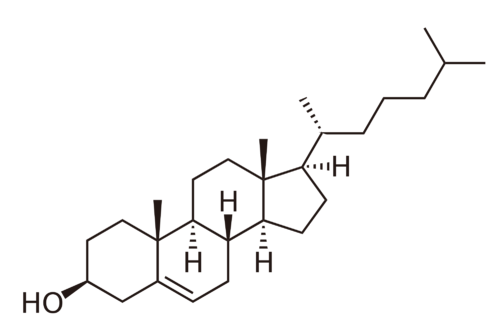
What does cholesterol really look like?
::胆固醇长什么样?We can write the structure of the cholesterol molecule a couple of different ways. The simplest approach is to just write C 27 H 46 O. This “structure” is not very useful because it does not tell us how the carbons, hydrogens, and oxygen are connected to one another. The structure in the figure above is much more helpful – we see how the different atoms are connected together to form the molecule.
::我们可以用几种不同的方式写出胆固醇分子的结构。 最简单的方法就是写C27H46O。 这个“结构”没有多大用处,因为它没有告诉我们碳、氢和氧是如何相互连接的。 上图中的结构更有用 — — 我们可以看到不同原子是如何连接在一起形成分子的。Lewis Electron-Dot Structures
::Lewis 电子-点结构In a previous chapter, you learned that the of an can be shown in a simple way with an electron dot diagram . A hydrogen atom is shown as H• because of its one valence electron. The structures of molecules that are held together by can be diagrammed by Lewis electron-dot structures . The hydrogen molecule is shown in Figure .
::在前一章中,你学会了可以用电子点图简单显示一个原子。氢原子因其一个valence 电子被显示为 H 。由静电点结构组合在一起的分子结构可以通过 Lewis 电子点结构图解。氢分子在图中显示。On the left is a single hydrogen atom with one electron. On the right is an H 2 molecule showing the electron cloud overlap.
::左侧是一个单氢原子,有一个电子。右侧是一个H2分子,显示电子云重叠。The shared pair of electrons is shown as two dots in between the two H symbols (H:H). This is called a single covalent bond , when two atoms are joined by the sharing of one pair of electrons. The single covalent bond can also be shown by a dash in between the two symbols (H–H). A structural formula is a formula that shows the arrangement of atoms in a molecule and represents covalent bonds between atoms by dashes.
::共享的对电子在两个H符号(H:H)之间显示为两个点。当两个原子与一对电子合在一起时,这被称为单一的共价债券。 单一的共价债券也可以通过两个符号(H-H)之间的破折来显示。 结构公式是一种公式,它显示分子中的原子的排列,并代表通过破折方式在原子之间的共价债券。The Octet Rule and Covalent Bonds
::章和共价债券When form, they conform to the by either losing or gaining electrons in order to achieve the electron configuration of the nearest . In a similar way, atoms share electrons in the formation of a covalent in bond such a way that each of the atoms involved in the bond can attain a noble- electron configuration. The shared electrons are “counted” for each of the atoms involved in the sharing. For hydrogen (H 2 ), the shared pair of electrons means that each of the atoms is able to attain the electron configuration of helium, the noble gas with two electrons. For atoms other than hydrogen, the sharing of electrons will usually provide each of the atoms with eight valence electrons.
::当组成时,它们会通过丢失或获取电子来达到最近的电子配置。同样地,原子在组成共价结合中分享电子,这样,债券所涉及的原子就能够达到高贵电子配置。共享电子对于分享所涉及的原子都是“计算”的。对于氢(H2)来说,共享电子对子意味着每个原子都能够实现的电子配置,高贵气体配有两种电子。对于氢以外的原子,共享电子通常将为每个原子提供八种价值电子。Summary
::摘要-
Lewis electron-dot structures show the bonding in covalent molecules.
::Lewis电子点结构显示共价分子的结合。 -
Covalent bonds between atoms can be indicated either with dots (
or a dash (-).
::可用点(或破折号(-)表示原子之间的共值联系。
Review
::回顾-
What is a single covalent bond?
::什么是单一的共价债券? -
How can covalently-bound atoms obey the octet rule?
::共生原子如何能服从信教规则? -
Does the hydrogen molecule obey the octet rule?
::氢分子遵守奥克特规则吗?
-
Lewis electron-dot structures show the bonding in covalent molecules.