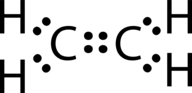9.7 多价共保债券
章节大纲
-
What do you do with your leftovers?
::你的剩菜怎么办?When working with covalent structures, it sometimes looks like you have leftover electrons. You apply the rules you learned so far and there are still some electrons hanging out there unattached. You can’t just leave them there. So where do you put them?
::当与共价结构一起工作时,有时看起来你还有剩余电子。你应用了迄今所学的规则,还有一些电子在外挂着。你不能把它们丢在那里。你把它们放在哪里?Multiple Covalent Bonds
::多价共价债券Some molecules are not able to satisfy the by making only single between the atoms. Consider the ethene, which has a of C 2 H 4 . The carbon atoms are bonded together, with each carbon also being bonded to two hydrogen atoms.
::有些分子无法通过在原子之间制造单一的原子来满足。 想想有C2H4的乙烯。 碳原子是结合在一起的, 每种碳也是结合到两个氢原子的。
::2个C原子=2×4=8 等值电子四 H原子=4×1=4 等值电子共12个分子中的等值电子If the Lewis dot structure was drawn with a single bond between the carbon atoms and with the octet rule followed, it would look like this:
::如果刘易斯点结构是用碳原子和所遵循的奥克特规则之间的单一联系来绘制的,它看起来是这样的:Incorrect dot structure of ethene. This Lewis structure is incorrect because it contains a total of 14 electrons. However, the Lewis structure can be changed by eliminating the lone pairs on the carbon atoms and having the share two pairs instead of only one pair.
::这个 Lewis 结构是不正确的, 因为它总共包含14个电子。 但是, Lewis 结构可以通过消除碳原子上的单对, 并且拥有两对而不是一对来改变。Correct dot structure for ethene. A double covalent bond is a covalent bond formed by atoms that share two pairs of electrons. The double covalent bond that occurs between the two carbon atoms in ethane can also be represented by a structural formula and with a molecular model as shown in Figure .
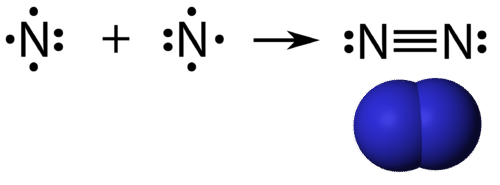
::双倍共价保证金是指由分享两对电子的原子组成的共价保证金。 乙烷中两个碳原子之间的双倍共价保证金也可以用结构公式和图所示的分子模型来表示。(A) The structural model for C2H4 consists of a double covalent bond between the two carbon atoms and single bonds to the hydrogen atoms. (B) Molecular model of C2H4. A triple covalent bond is a covalent bond formed by atoms that share three pairs of electrons. The nitrogen is a that composes the majority of Earth’s atmosphere. A nitrogen has five , which can be shown as one pair and three single electrons. When combining with another nitrogen atom to form a diatomic molecule , the three single electrons on each atom combine to form three shared pairs of electrons.
::三重共价联结是由原子组成的共价联结,原子共有三对电子。氮构成地球大气的大部分。氮有五对,可以显示为一对和三个单一电子。 当结合另一个氮原子形成一个解剖分子时,每个原子的三个单电子组合成三对共享电子。Triple bond in N2. Each nitrogen atom follows the octet rule with one lone pair of electrons and six electrons that are shared between the atoms.
::每个氮原子都遵循八点规则 单对电子 和六个电子 由原子共享Summary
::摘要-
Lewis structures can be drawn for molecules that share multiple pairs of electrons.
::刘易斯结构可以用来绘制与多个电子对共享的分子。
Review
::回顾-
Why is the first ethene Lewis structure incorrect?
::为什么第一种Ethene Lewis结构不正确? -
What do the single electrons in nitrogen do to form a triple bond?
::氮中的单电子会如何形成三重结合? -
Draw the Lewis structure for ethyne C
2
H
2
.
::为 ethyne C2H2 绘制 Lewis 结构。
-
Lewis structures can be drawn for molecules that share multiple pairs of electrons.