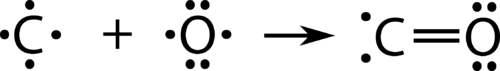9.8 协调共价债券
章节大纲
-
Is sharing a good thing?
::分享是好事吗?Remember when you were younger and were told to share your favorite toy with your brother or sister or friend? You probably didn’t want to share, but did anyway. It turned out that you had more fun playing with the toy together than if you had kept it to yourself. Atoms also have to share what’s theirs with another that has nothing to contribute to the situation. But the end result is a new structure.
::记得你年轻时曾被告知要与你的兄弟或姐妹或朋友分享你最喜欢的玩具吗?你可能不想分享,但还是做到了。 结果发现,你与玩具玩在一起比与自己独享更好玩。 原子也不得不与对情况毫无贡献的其他人分享他们的东西。 但最终结果是一个新的结构。Coordinate Covalent Bonds
::协调共价债券Each of the that we have looked at so far has involved each of the atoms that are bonding contributing one of the electrons to the shared pair. There is an alternate type of covalent bond in which one of the atoms provided both of the electrons in a shared pair. Carbon monoxide, CO, is a toxic that is released as a by-product during the burning of fossil fuels. The bonding between the C atom and the O atom can be thought of as proceeding in this way.
::我们迄今所观察的每一个原子都涉及每个原子,这些原子正在将一个电子与共享的对子连接起来。 有一种替代的共价连接,其中一种原子在共享的对子中提供两种电子。 二氧化碳是一种有毒物质,在燃烧化石燃料期间作为一种副产品释放出来。 C原子与O原子的连接可以被视为以这种方式进行。Formation of a CO double bond (incorrect structure).
::CO双倍保证金的形成(结构不正确)。At this point, a double bond has formed between the two atoms, with each atom providing one of the electrons to each bond. The oxygen atom now has a stable octet of electrons, but the carbon atom only has six electrons and is unstable. This situation is resolved if the oxygen atom contributes one of its lone pairs in order to make a third bond with the carbon atom.
::在这一点上,两个原子之间形成了一种双重联系,每个原子都向每个连接提供一种电子。 氧原子现在拥有稳定的电离值,但碳原子只有六种电子,不稳定。 如果氧原子贡献其独一一对,从而与碳原子建立第三个联系,这种情况就解决了。Correct CO structure.
::正确的CO结构。The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. One of the bonds is a coordinate covalent bond , a covalent bond in which one of the atoms contributes both of the electrons in the shared pair.
::一氧化碳分子由碳原子和氧原子之间的三重共价联系正确代表。 其中一种联系是协调共价联系,一种共价联系,一种共价联系,其中一原子在共享对子中贡献了两种电子。Once formed, a coordinate covalent bond is the same as any other covalent bond. It is not as if the two conventional bonds in the CO molecule are stronger or different in any other way than the coordinate covalent bond.
::协调共价债券一旦形成,与任何其他共价债券一样。 这并不是说CO分子中两种常规债券比协调共价债券更强大或不同。Summary
::摘要-
Coordinate covalent bonds can form when one atom provides a lone pair of electrons to the bond.
::当一个原子为该债券提供单对电子时,可形成坐标共价债券。 -
Coordinate covalent bonds are as strong as other covalent bonds.
::协调共价债券与其他共价债券一样强大。
Review
::回顾-
Where does the third covalent bond in the CO molecule come from?
::CO分子的第三个共价联系来自何处? -
Why is the incorrect structure for CO above wrong?
::为什么CO的错误结构高于CO的错误结构? -
Are coordinate covalent bonds stronger or weaker than regular covalent bonds?
::协调共价债券是否比正常共价债券更强或更弱?
-
Coordinate covalent bonds can form when one atom provides a lone pair of electrons to the bond.