9.10 共振
章节大纲
-
Is this a reflection or a doorway ?
::这是反射还是门道?We might look at the picture above and think we are looking at the image of a room as reflected in a mirror (and we probably are). But we can crop the picture in such a way as to give the impression we are looking at the real room through a door. We would see the same thing and receive the same information, but it would be from a different perspective. There are molecules that can be represented in different ways and reality becomes a matter of interpretation.
::我们也许可以看看上面的图片,并且认为我们是在看镜子中反映的一个房间的图像(我们也许正在看 ) 。 但是,我们可以通过门来给人一个印象,让我们看到真实的房间。我们会看到同样的事情,并获得同样的信息,但从不同的角度看。有些分子可以以不同的方式表达,而现实则变成一个解释问题。Resonance
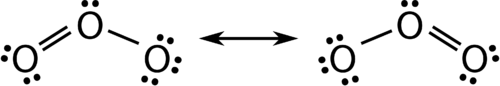
::共振There are some cases in which more than one viable Lewis structure can be drawn for a molecule. An example is the ozone (O 3 ) molecule in Figure . There are a total of 18 electrons in the structure and so the following two structures are possible.
::有些情况下,可以为分子绘制一个以上可行的刘易斯结构,例如图3中的臭氧(O3)分子。结构中共有18个电子,因此以下两种结构是可能的。Resonance forms of ozone.
::臭氧的共振形式。The structure on the left (see Figure ) can be converted to the structure on the right by a shifting of electrons without altering the positions of the atoms.
::左侧的结构(见图)可以通过电子转移而转换成右侧的结构,而不改变原子的位置。It was once thought that the structure of a molecule such as O 3 consisted of one single bond and one double bond which then shifted back and forth as shown above. However, further studies showed that the two bonds are identical. Any double covalent bond between two given atoms is typically shorter than a single covalent bond . Studies of the O 3 and other similar molecules showed that the bonds were identical in length. Interestingly, the length of the bond is in between the lengths expected for an O-O single bond and a double bond.
::曾一度认为,O3这样的分子的结构由单一的保证金和双倍的保证金组成,然后如上所示向后转移,然而,进一步的研究显示,这两种保证金是相同的,两个给定原子之间的任何双重共价保证金通常比单一共价保证金短。对O3和其他类似的分子的研究显示,这些保证金长度相同。有趣的是,保证金的长度在O-O单保证金和双倍保证金预期的长度之间。Resonance is the use of two or more Lewis structures to represent the covalent bonding in a molecule. One of the valid structures is referred to as a resonance structure. It is now understood that the true structure of a molecule which displays resonance is that of an average or a hybrid of all the resonance structures. In the case of the O 3 molecule, each of the between O atoms is best thought of as being “one and a half” bonds, as opposed to either a pure single bond or a pure double bond. This “half-bond” can be shown as a dotted line in both the Lewis structure and the molecular model (see Figure ).
::共振是指使用两个或两个以上的 Lewis 结构来代表分子中的共价结合。一个有效的结构被称为共振结构。现在人们的理解是,显示共振结构的分子的真正结构是平均或所有共振结构的混合体。在O3分子的情况中,O原子之间的每一种都最好被认为是“一个半”的联结,而不是纯单一的联结或纯的双重联结。在刘易斯结构和分子模型中,这种“半瓶”都可以作为点线显示(见图 )。“Half-bond” model of ozone molecule.
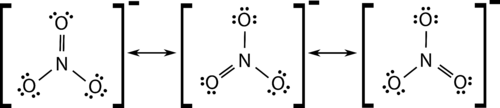
::臭氧分子“半瓶”模型。Many polyatomic also display resonance. In some cases, the true structure may be an average of three valid resonance structures, as in the case of the nitrate ion, NO 3 − (see Figure ).
::在许多情况下,真实的结构可能是三种有效的共振结构,例如硝酸盐离子,NO3-(见图)。Resonance structure of nitrate anion.
::硝酸盐阴离子的共振结构。The bond lengths between the central N and each O atom are identical and the bonds can be approximated as being equal to one and one-third bonds.
::中央N和每个O原子之间的债券长度相同,债券可大致相当于1和1/3债券。Summary
::摘要-
Resonance structures are averages of different Lewis structure possibilities.
::共振结构是不同刘易斯结构可能性的平均数。 -
Bond lengths are intermediate between covalent single bonds and covalent double bonds.
::债券长度介于共价单债券和共价双面债券之间。
Review
::回顾-
How many electrons total are in the ozone structure?
::臭氧结构中总共有多少电子? -
What is changed in the two resonance structures of ozone?
::臭氧的两个共振结构有什么变化? -
How can we think of the covalent bonds in ozone?
::我们怎样才能想到臭氧中的共价联结?
-
Resonance structures are averages of different Lewis structure possibilities.