9.11 《规则》的例外
章节大纲
-
Are rules always followed?
::规则是否一直得到遵守?Every spring, millions of Americans file their income tax forms. The different rules determine how much tax a person pays. There are also exceptions to the rules. You pay less tax if you are married and/or have children. There are certain limits on how much money you can make before paying taxes. The rule is that you pay taxes, but there are also exceptions based on your personal situation. The bonding rules for molecules are generally applicable, but there are some exceptions allowed.
::每年春天,数百万美国人提交所得税表格。不同的规则决定一个人缴纳的税额。也有例外。如果已婚和/或有子女,缴纳的税额会减少。在缴纳税前可以挣多少金额有一定的限制。规则是缴纳税,但根据个人情况也有例外。分子的结合规则是普遍适用的,但也允许一些例外。Exceptions to the Octet Rule
::原《规则》的例外As the saying goes, all rules are made to be broken. When it comes to the , that is true. Exceptions to the octet rule fall into one of three categories: (1) an incomplete octet , (2) odd- molecules , and (3) an expanded octet.
::俗话说,所有的规则都是被打破的。当谈到 , 确实如此。 信条规则的例外分为三类1) 不完整的信条,(2) 奇分子,(3) 扩大信条。
Incomplete Octet
::不完整的奥本In some compounds, the number of electrons surrounding the central in a stable molecule is fewer than eight. Beryllium is an and so may be expected to form . However, its very small size and somewhat higher ionization energy compared to other actually lead to beryllium forming primarily . Since beryllium only has two , it does not typically attain an octet through sharing of electrons. The Lewis structure of gaseous beryllium hydride (BeH 2 ) consists of two single between Be and H (see Figure ).
::在一些化合物中,在稳定分子中,中央周围的电子数量不到8个。预计会形成,因此可能形成。然而,其体积很小,离子化能量比其他化合物稍高一些,实际上主要导致的形成。由于只有两个,因此通常不会通过分享电子而达到八点。气态氢(BeH2)的刘易斯结构由Be和H之间的两个单一组成(见图2)。Beryllium hydride.
::氢化。Boron and aluminum, with three valence electrons, also tend to form covalent compounds with an incomplete octet. The central boron atom in boron trichloride (BCl 3 ) has six valence electrons as shown in Figure .
::如图3所示,三氯化(BCl3)的中子子原子有六种等值电子。Boron trichloride.
::敌百虫Odd-Electron Molecules
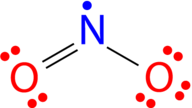
::Odd- 电动分子There are a number of molecules whose total number of valence electrons is an odd number. It is not possible for all of the atoms in such a molecule to satisfy the octet rule. An example is nitrogen dioxide (NO 2 ). Each oxygen atom contributes six valence electrons and the nitrogen atom contributes five for a total of seventeen. The lewis structure for NO 2 appears in Figure .
::有一些分子的总值电子数是一个奇数。 无法让这种分子中的所有原子都满足八溴二苯醚规则。 例如二氧化氮( NO2) 。 每个氧原子共贡献六种值电子, 氮原子共贡献五种17种。 图中显示了二氧化氮的结构 。The Lewis structure for nitrogen dioxide, an odd electron molecule.
::二氧化碳的刘易斯结构 一种奇异的电子分子Expanded Octets
::扩展日记Atoms of the second period cannot have more than eight valence electrons around the central atom. However, atoms of the third period and beyond are capable of exceeding the octet rule by having more than eight electrons around the central atom. Starting with the third period, the d sublevel becomes available, so it is possible to use these in bonding, resulting in an expanded octet.
::第二个周期的原子在中央原子周围不能有超过8个等值电子。 但是,第三个周期及以后的原子在中央原子周围拥有8个以上的电子,因此能够超过八个以上奥氏定律。 从第三个周期开始,D子层可以使用,因此可以在结合中使用这些电源,从而导致一个扩大的奥氏。Phosphorus and sulfur are two that react with halogen elements and make stable compounds with expanded octets. In phosphorus pentachloride, the central phosphorus atom makes five single bonds to chlorine atoms and as a result has ten electrons surrounding it (see Figure ). In sulfur hexafluoride, the central sulfur atom has twelve electrons from its six bonds to fluorine atoms (see Figure ).
::磷和硫是两种与卤素元素发生反应并用扩大的八氯化物形成稳定化合物的化合物,在五氯化磷中,中磷原子与氯原子形成5个单一的链条,因此周围有10个电子(见图 ) 在六氟化硫中,中硫原子从6个链条到氟原子有12个电子(见图 )。Phosphorus pentachloride. Left image: Lewis structure Right image: molecular model
::五氯化磷磷磷、左图象:刘易斯结构 右图象:分子模型Sulfur hexafluoride. Left image: Lewis structure Right image: molecular model.
::六氟化硫,左图象:刘易斯结构,右图象:分子模型。Summary
::摘要-
Exceptions exist to the rules for covalent bonding.
::共价结合规则有例外。 -
These exceptions apply to atoms whose electrons will not accommodate the normal octet rule.
::这些例外适用于电子无法适应正常奥克特规则的原子。
Review
::回顾-
What is an incomplete octet?
::什么是不完整的奥斯特? -
What is an odd-electron molecule?
::什么是奇电子分子? -
Why are there extra electrons in the expanded octet?
::为什么在扩展的奥斯特内 会有额外的电子呢?
-
Exceptions exist to the rules for covalent bonding.