9.15 分子形状:中原子上的孤独对等
章节大纲
-
How can all these clothes fit into such a small space?
::这些衣服怎么能装在这么小的空间里?When we travel, we often take a lot more stuff than we need. Trying to fit it all in a suitcase can be a real challenge. We may have to repack or just squeeze it all in. Atoms often have to rearrange where the electrons are in order to create a more stable structure.
::当我们旅行时,我们常常需要更多的东西。试图把它们都装在手提箱里可能是一个真正的挑战。我们可能不得不重新包装或挤压它。原子往往不得不重新排列电子的位置,以便创造一个更稳定的结构。Central Atom with One or More Lone Pairs
::中心原子,一个或数个单对子The molecular geometries of molecules change when the central has one or more lone pairs of electrons. The total number of pairs, both bonding pairs and lone pairs, leads to what is called the electron domain geometry. When one or more of the bonding pairs of electrons is replaced with a lone pair , the molecular geometry (actual shape) of the molecule is altered. In keeping with the A and B symbols established in the previous section, we will use E to represent a lone pair on the central atom (A). A subscript will be used when there is more than one lone pair. Lone pairs on the surrounding atoms (B) do not affect the geometry.
::当中央拥有一个或多个单对电子时分子的分子几何变化。 双对的总数, 包括双对和单对, 导致所谓的电子域几何。 当一个或数对连接的电子被单对替换时, 分子的分子几何( 实际形状) 被改变。 根据上一节确定的 A 和 B 符号, 我们将使用 E 代表中央原子的单对( A ) 。 当有不止一对单对时, 将使用下标 。 周围原子的单对( B) 不影响几何 。AB 3 E: Ammonia, NH 3
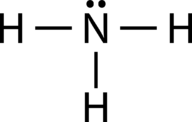
::AB3E:氨、NH3The ammonia molecule contains three single bonds and one lone pair on the central nitrogen atom (see Figure ).
::氨分子在中央氮原子上含有3个单键和1对单对(见图 )。Lone pair electrons in ammonia.
::氨水中的单对电子。The domain geometry for a molecule with four electron pairs is tetrahedral, as was seen with CH 4 . In the ammonia molecule, one of the electron pairs is a lone pair rather than a bonding pair. The molecular geometry of NH 3 is called trigonal pyramidal (see Figure ).
::四对电子分子的域几何是四面形的,如CH4所见。在氨分子中,电子对之一是单对,而不是联结对。NH3的分子几何称为三角形金字塔(见图 ) 。Ammonia molecule.
::氨分子。Recall that the bond angle in the tetrahedral CH 4 molecule is 109.5°. Again, the replacement of one of the bonded electron pairs with a lone pair compresses the angle slightly. The H-N-H angle is approximately 107°.
::回顾四面式CH4分子中的结合角为109.5°。 再一次,用单对电子对替换一个捆绑式电子对对使角稍微压缩。 H-N-H角约为107°。AB 2 E 2 : Water, H 2 O
::AB2E2:水,H2OA water molecule consists of two bonding pairs and two lone pairs (see Figure ).
::水分子由两对连接对和两对单对组成(见图 )。Lone pair electrons on water.
::单对电子在水上。As for methane and ammonia, the domain geometry for a molecule with four electron pairs is tetrahedral. In the water molecule, two of the electron pairs are lone pairs rather than bonding pairs. The molecular geometry of the water molecule is bent. The H-O-H bond angle is 104.5°, which is smaller than the bond angle in NH 3 (see Figure ).
::至于甲烷和氨,四对电子分子的域数是四面形。在水分子中,两对电子是单对,而不是联结对。水分子的分子数是弯曲的。H-O-H的介质角为104.5°,小于NH3中的联结角(见图 )。Water molecule.
::水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子 水分子AB 4 E: Sulfur Tetrafluoride, SF 4
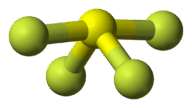
::AB4E:四氟化硫,SF4The Lewis structure for SF 4 contains four single bonds and a lone pair on the sulfur atom (see Figure ).
::SF4的刘易斯结构包含4个单一债券和硫磺原子上的单对(见图 )。Lone pair electrons in SF 4 .
::SF4中的单对电子。The sulfur atom has five electron groups around it, which corresponds to the trigonal bipyramidal domain geometry, as in PCl 5 (see Figure ). Recall that the trigonal bipyramidal geometry has three equatorial atoms and two axial atoms attached to the central atom. Because of the greater repulsion of a lone pair, it is one of the equatorial atoms that are replaced by a lone pair. The geometry of the molecule is called a distorted tetrahedron or seesaw.
::硫磺原子周围有五个电子组,这与PCl5(见图)中的三角双极相交域域几何法相对应。回顾三角双极相交几何法有三个赤道原子和两个轴向原子与中原子相连。由于单方对子的反射较大,它是被单方对子取代的赤道原子之一。分子的几何法被称为扭曲的四面形或锯锯。Ball and stick model for SF 4 .
::SF4的球和棒型Geometries in Which the Central Atom Has One or More Lone Pairs Total Number of Electron Pairs Number of Bonding Pairs Number of Lone Pairs Electron Domain Geometry Molecular Geometry Examples 3 2 1 trigonal planar bent O 3 4 3 1 tetrahedral trigonal pyramidal NH 3 4 2 2 tetrahedral bent H 2 O 5 4 1 trigonal bipyramidal distorted tetrahedron (seesaw) SF 4 5 3 2 trigonal bipyramidal T-shaped CIF 3 5 2 3 trigonal bipyramidal linear I 3 - 6 5 1 octahedral square pyramidal BrF 5 6 4 2 octahedral square planar XeF 4 Summary
::摘要-
The presence of lone pair electrons influences the three-dimensional shape of the molecule.
::单对电子的存在 影响分子的三维形状
Review
::回顾-
Why does water have a bent geometry?
::为什么水有弯曲几何? -
Why is ammonia not a planar molecule?
::为什么氨不是平板分子? -
How would we write the configuration for xenon tetrafluoride using the ABE system?
::我们如何使用ABE系统写出 xenon 四氟化物的配置?
-
The presence of lone pair electrons influences the three-dimensional shape of the molecule.