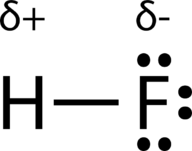9.16 债券极地
章节大纲
-
What makes people share?
::是什么使人们分享?Have you ever spent time with someone you really didn’t like? You had nothing in common with them and did not want to have anything to do with them. On the other hand, there are people you enjoy being with. You have a lot in common and like to share with them. Atoms work the same way. If there are strong differences in their attraction of electrons, one atoms gets the electrons and the other loses them. If they are similar, they share the electrons to form a .
::你曾经和你真正不喜欢的人在一起过吗?你与他们没有任何共同之处,也不想和他们有任何关系。另一方面,你喜欢和一些人在一起。你有很多共同之处,也喜欢和他们分享。原子同样工作方式。如果在电子的吸引力上存在很大的差异,一个原子会得到电子,另一个原子会失去电子。如果它们相似,它们就会分享电子形成电子。Bond Polarity
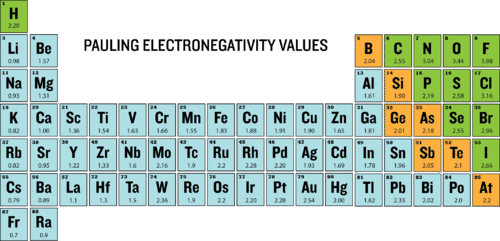
::债券极度Electronegativity is defined as the ability of an atom to attract electrons when the atoms are in a . Electronegativities of are shown in the periodic table below.
::电能被定义为原子处于.电能状态时原子吸引电子的能力。 电能如下表所示。Electronegativities of elements.
::元素的电能。The degree to which a given bond is ionic or covalent is determined by calculating the difference in electronegativity between the two atoms involved in the bond.
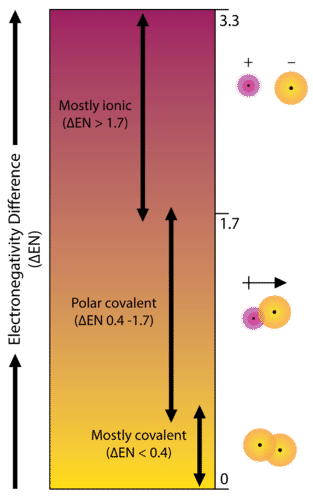
::确定某一保证物的离子或共价的程度,是计算该保证物所涉及的两个原子之间电子能量差。As an example, consider the bond that occurs between an atom of potassium and an atom of fluorine. Using the table, the difference in electronegativity is equal to 4.0 - 0.8 = 3.2. Since the difference in electronegativity is relatively large, the bond between the two atoms is ionic. Since the fluorine atom has a much larger attraction for electrons than the potassium atom does, the from the potassium atom is completely transferred to the fluorine atom. The diagram below shows how difference in electronegativity relates to the ionic or covalent character of a .
::例如,考虑到钾原子和氟原子之间的关联。使用表格,电子能量差异等于4.0-0-0.8=3.2。由于电子能量差异相对较大,两个原子之间的关联是离子的。由于氟原子对电子的吸引力比钾原子大得多,因此从钾原子到氟原子的吸引力完全转移到了氟原子。下图显示了电子能量差异与一个原子的离子或共价特性的关系。Bond type is predicated on the difference in electronegativity of the two elements involved in the bond.
::债券类型取决于债券所涉两个要素在电子能力方面的差异。Non-polar Covalent Bonds
::非极共价债券A bond in which the electronegativity difference is less than 1.7 is considered to be mostly covalent in character. However, at this point we need to distinguish between two general types of covalent bonds. A non- polar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two atoms. In a non-polar covalent bond, the distribution of electrical charge is balanced between the two atoms.
::电子效应差小于1.7的债券被认为在性质上主要是共价,然而,在这一点上,我们需要区分两种一般的共价债券。非极价共价债券是一种共价债券,在这两种原子之间平等分享连接电子。在非极价共价债券中,电费的分配在两种原子之间是平衡的。A nonpolar covalent bond is one in which the distribution of electron density between the two atoms is equal.
::非极价共价债券是指两个原子之间电子密度分布相等的债券。The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the density surrounding the Cl 2 molecule is symmetrical. Also note that molecules in which the electronegativity difference is very small (<0.4) are also considered non-polar covalent. An example would be a bond between chlorine and bromine .
::两个氯原子在单共价联结中平等分享对电子,而Cl2分子的密度是对称的。还注意到,电子率差异很小的分子(<0.4)也被认为是非极共价,例如氯和溴之间的联结(EN=3.0-2.8=0.2)。Polar Covalent Bonds
::极共价债券A bond in which the electronegativity difference between the atoms is between 0.4 and 1.7 is called a polar covalent bond. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal. In a polar covalent bond, sometimes simply called a polar bond, the distribution of electrons around the molecule is no longer symmetrical.
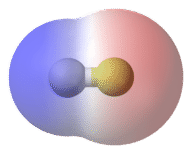
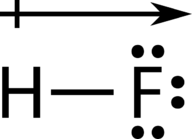
::原子之间的电子效应差异在0.4和1.7之间的一种联系被称为极性共价债券。 极性共价债券是一种共价债券,其中原子对电子的吸引力不平等,因此分享是不平等的。 在极性共价债券中,有时简单地称为极性债券,分子周围电子的分布不再是对称的。In the polar covalent bond of HF, the electron density is unevenly distributed. There is a higher density (red) near the fluorine atom, and a lower density (blue) near the hydrogen atom.
::在高频极共价联结中,电子密度分布不均,氟原子附近密度较高(红色),氢原子附近密度较低(蓝色)。An easy way to illustrate the uneven electron distribution in a polar covalent bond is to use the Greek letter delta .
::使用希腊字母三角洲()是说明极地共价债券电子分布不均的简单方法。Use of δ to indicate partial charge.
::使用__________表示部分收费。The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser electronegativity acquires a partial positive charge. The delta symbol is used to indicate that the quantity of charge is less than one. A crossed arrow can also be used to indicate the direction of greater electron density.
::电子密度较大的原子获得部分负电荷,而电子密度较小的原子获得部分正电荷。三角洲符号用来表示充电量少于1。横箭也可以用来表示电子密度较大的方向。Use of crossed arrow to indicate polarity.
::使用横箭表示两极性。If it wasn't for our understanding of bond polarity, we'd have a really tough time cleaning our clothing. Use this simulation to understand how the unique of soap molecules helps to clean our clothing!
::如果不是因为我们理解联结的两极性, 我们就会有一个非常艰难的时间来清理衣服。 使用这个模拟来理解肥皂分子的独特性 是如何帮助我们清洗衣服的!Summary
::摘要-
The electronegativity of an atom determines how strongly it attracts electrons to itself.
::原子的电子性决定了它本身吸引电子的强度。 -
The polarity of a bond is affected by the electronegativity values of the two atoms involved in that bond.
::债券的两极性受到该债券所涉及的两个原子的电子浓缩值的影响。
Review
::回顾-
If two atoms bonded together have an electronegativity difference of 1.9, what is
the bond type?
::如果两个原子捆绑在一起 电子能量差异为1.9 那么债券类型是什么? -
What would be the bond type for BH
2
?
::BH2的债券类型是什么? -
Your friend tells you that the LiF bond is covalent.
Are they correct? Why or why not.
::你的朋友告诉你,LiF债券是共价的,它们正确吗?
-
The electronegativity of an atom determines how strongly it attracts electrons to itself.