9.22 混合轨道
章节大纲
-
Do you recognize this plant?
::你认得这植物吗?If we were walking on the beach, the plants shown above would look very different. They would be short and sticking out of the sand. When we see them this way, we do not immediately recognize them as beach plants. Often we need to look at the world around us in different ways to understand things better.
::如果我们在海滩上行走,上面显示的植物看起来会非常不同。它们会很短,会从沙地上露出。当我们这样看待它们时,我们并不立即认出它们是海滩植物。我们常常需要以不同的方式看待我们周围的世界,以便更好地了解事情。Hybrid Orbitals – sp 3
::混合轨道轨道 - sp3The bonding scheme described by must account for molecular geometries as predicted by VSEPR theory . To do that, we must introduce a concept called hybrid orbitals .
::由VSEPR理论所预测的分子几何必须由VSEPR理论所描述的联结计划来解释。 要做到这一点,我们必须引入一个称为混合轨道的概念。sp 3 Hybridization
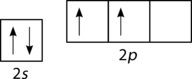
::spec3 混合化Unfortunately, overlap of existing atomic ( s , p , etc.) is not sufficient to explain some of the bonding and molecular geometries that are observed. Consider the carbon and the methane (CH 4 ) molecule. A carbon has the electron configuration of 1s 2 2s 2 2p 2 , meaning that it has two unpaired electrons in its 2p orbitals, as shown in Figure .
::不幸的是,现有原子(s, p, etc.)的重叠不足以解释所观测到的一些联结和分子几何。考虑碳和甲烷(CH4)分子。碳的电子配置为1s2 2s2 2p2,这意味着其2p轨道上有两个未受保护的电子,如图所示。Orbital configuration for carbon atom.
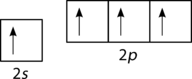
::碳原子轨道配置。According to the description of valence bond theory so far, carbon would be expected to form only two bonds, corresponding to its two unpaired electrons. However, methane is a common and stable molecule, with four equivalent C−H bonds. To account for this, one of the 2s electrons is promoted to the empty 2p orbital (see Figure ).
::根据迄今对物价债券理论的描述,预计碳只构成两个债券,相当于两个未受污染的电子,然而,甲烷是一个常见和稳定的分子,有四个等效的C-H债券,为此,2个电子中的1个被提升到空的2p轨道(见图 )。Promotion of carbon s electron to empty p orbital.
::将碳电子推广到空的轨道轨道。Now, four bonds are possible. The promotion of the “costs” a small amount of energy , but recall that the process of bond formation is accompanied by a decrease in energy. The two extra bonds that can now be formed results in a lower overall energy and thus greater stability to the CH 4 molecule. Carbon normally forms four bonds in most of its compounds.
::现在,四个债券是可能的。 推广“成本”的“成本”只是一小部分能量,但记得债券形成过程伴随着能源的减少。 现在可以形成的另外两个债券导致整体能量降低,从而增强CH4分子的稳定性。 碳通常在其大部分化合物中形成四个债券。The number of bonds is now correct, but the geometry is wrong. The three p orbitals (p x , p y , p z ) are oriented at 90 o relative to one another. However, as was seen from VSEPR theory, the observed H−C−H bond angle in the tetrahedral CH 4 molecule is actually 109.5 o . Therefore, the methane molecule cannot be adequately represented by simple overlap of the 2s and 2p orbitals of carbon with the 1s orbitals of each hydrogen atom.
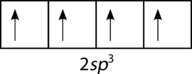
::现在债券的数量是正确的,但几何是错误的。 三个轨道( px, py, pz) 的定位是相对的90o。 然而,从VSEPR理论可以看出,四面CH4分子中观察到的H-C-H债券角度实际上为109.5o。 因此,甲烷分子无法通过碳的2和2p轨道与每个氢原子的1s轨道的简单重叠来充分体现。To explain the bonding in methane, it is necessary to introduce the concept of hybridization and hybrid atomic orbitals. Hybridization is the mixing of the atomic orbitals in an atom to produce a set of hybrid orbitals. When hybridization occurs, it must do so as a result of the mixing of nonequivalent orbitals. In other words, s and p orbitals can hybridize but p orbitals cannot hybridize with other p orbitals. Hybrid orbitals are the atomic orbitals obtained when two or more nonequivalent orbitals form the same atom combine in preparation for bond formation. In the current case of carbon, the single 2s orbital hybridizes with the three 2p orbitals to form a set of four hybrid orbitals, called sp 3 hybrids (see Figure ).
::为了解释甲烷的结合,有必要引入混合和混合原子轨道的概念。混合是指将原子轨道混合在一个原子中产生一套混合轨道。当混合发生时,它必须作为非等值轨道混合的结果。换句话说,轨道和轨道可以混合,但轨道不能与其他轨道混合。混合轨道是当两个或两个以上非等值轨道结合成同一原子以准备形成联结时获得的原子轨道。在目前的碳方面,单2个轨道混合与3个2P轨道混合成一组4个混合轨道,称为螺旋3混合(见图)。Carbon sp 3 hybrid orbitals.
::碳 sp3 混合轨道。The sp 3 hybrids are all equivalent to one another. Spatially, the hybrid orbitals point towards the four corners of a tetrahedron (see Figure ).
::从空间上看,混合轨道指向四面形的四个角(见图 )。The process of sp 3 hybridization is the mixing of an s orbital with a set of three p orbitals to form a set of four sp 3 hybrid orbitals. Each large lobe of the hybrid orbitals points to one corner of a tetrahedron. The four lobes of each of the sp 3 hybrid orbitals then overlap with the normal unhybridized 1s orbitals of each hydrogen atoms to form the tetrahedral methane molecule.
::螺旋3混合化的过程是将一个轨道与一组三颗轨道相混合,形成一套四颗螺旋3混合轨道。混合轨道的每个大叶子都指向四面形的一个角。每颗螺旋3混合轨道的四叶子随后与每个氢原子的正常非节制轨道1s相重叠,形成四面体甲烷分子。Summary
::摘要-
Electrons hybridize in order to form covalent bonds.
::电子混合,以形成共价债券。 -
Nonequivalent orbitals mix to form hybrid orbitals.
::非等效轨道混合构成混合轨道。
Review
::回顾-
Why is carbon expected to form only two covalent bonds?
::为什么预计碳只构成两个共价债券? -
How many covalent bonds does carbon actually form?
::碳实际形成多少共价债券? -
What needs to happen to allow carbon to form four bonds?
::需要做些什么才能使碳形成四份债券?
-
Electrons hybridize in order to form covalent bonds.