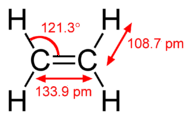9.24 Sigma和Pi债券
章节大纲
-
How many people do you think are squeezed on this street?
::你觉得这条街上有多少人被挤压?Our minds can handle two electrons interacting with one another in a sphere of space. But then we start putting in double bonds and triple bonds. The way we draw these bonds on paper suggests we are squeezing more electrons into the same space, and that doesn’t work. Electrons don’t like to be pushed together (especially since they all have negative charges that repel one another). So we need a more complex picture that works for all these electrons.
::我们的头脑可以处理两个电子在空间领域相互互动的问题。 但是,我们开始设置双倍债券和三倍债券。 我们用纸面方式提取这些债券的方式表明,我们正在将更多的电子挤进同一个空间,这行不通。 电人不喜欢被一起推(特别是因为他们都有相互反射的负电荷 ) 。 因此我们需要一张更复杂的图片,为所有这些电子工作。Sigma and Pi Bonds
::Sigma和Pi债券The hybridization model helps explain molecules with double or triple bonds (see Figure ). Ethene (C 2 H 4 ) contains a double covalent bond between the two carbon atoms and single bonds between the carbon atoms and the hydrogen atoms. The entire molecule is planar.
::混合化模型有助于解释双倍或三倍联结的分子(见图)。Ethene(C2H4)含有两个碳原子之间的双倍共价联结,以及碳原子和氢原子之间的单一联结。整个分子是平面。Geometry of ethene molecule.
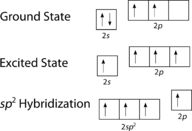
::乙烯分子的几何。As can be seen in Figure , the electron domain geometry around each carbon independently is trigonal planar. This corresponds to sp 2 hybridization. Previously, we saw carbon undergo sp 3 hybridization in a CH 4 molecule, so the promotion is the same for ethene, but the hybridization occurs only between the single s and two of the three p orbitals. Thus generates a set of three sp 2 hybrids along with an unhybridized 2p z orbital. Each contains one electron and so is capable of forming a .
::从图中可以看出,每个独立碳周围的电子域几何是三角平面。 这相当于 sp2 混合化 。 以前,我们看到碳在CH4 分子中经历 sp3 混合化, 所以对乙烯的促销是一样的, 但混合化只在单星和三个轨道中的两个轨道之间发生。 因此, 产生一组三颗 sp2 混合体, 加上一个未节制的 2pz 轨道。 每个轨道都包含一个电子, 因此能够形成一个 。Hybridization in ethene.
::乙烯的混合。The three sp 2 hybrid orbitals lie in one plane, while the unhybridized 2p z orbital is oriented perpendicular to that plane. The bonding in C 2 H 4 is explained as follows. One of the three sp 2 hybrids forms a bond by overlapping with the identical hybrid orbital on the other carbon . The remaining two hybrid orbitals form bonds by overlapping with the 1s orbital of a hydrogen atom. Finally, the 2p z orbitals on each carbon atom form another bond by overlapping with one another sideways.
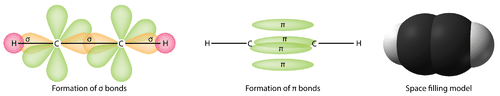
::3个螺旋2混合轨道位于一平面上,而未节制的2pz轨道方向与该平面垂直。C2H4的结合解释如下:3个螺旋2混合体之一与其他碳的相同混合轨道重叠,形成联系。其余两个混合轨道形成联系,与氢原子的1号轨道重叠。最后,每个碳原子上的2pz轨道形成另一个联系,与另一个侧面重叠。It is necessary to distinguish between the two types of covalent bonds in a C 2 H 4 molecule. A sigma bond ( bond) is a bond formed by the overlap of orbitals in an end-to-end fashion, with the electron density concentrated between the nuclei of the bonding atoms. A pi bond ( bond) is a bond formed by the overlap of orbitals in a side-by-side fashion with the electron density concentrated above and below the plane of the nuclei of the bonding atoms. Figure shows the two types of bonding in C 2 H 4 . The sp 2 hybrid orbitals are purple and the p z orbital is blue. Three sigma bonds are formed from each carbon atom for a total of six sigma bonds total in the molecule. The pi bond is the “second” bond of the double bonds between the carbon atoms and is shown as an elongated green lobe that extends both above and below the plane of the molecule. This plane contains the six atoms and all of the sigma bonds.
::需要区分C2H4分子中两种共价债券。Sigma债券( 12) 是轨道重叠以端到端方式形成的一种债券, 电子密度集中在连接原子的核之间。 pi 债券是轨道重叠以平行方式与集中在连接原子的核平面以上和下方的电子密度重叠形成的一种债券。 图显示C2H4中的两种类型的联结。 螺旋2混合轨道是紫色的, 螺旋轨道是蓝色的。 每个碳原子组成了三个双面债券, 总共是分子中的6个西格玛债券。 pi 债券是碳原子之间双面链接的“ 第二” , 显示为分子平面以上和下方的长绿色链接。 此平面包含6个原子和所有西格玛债券。
Sigma and pi bonds.
::西格玛和皮债券In a conventional Lewis electron-dot structure, a double bond is shown as a double dash between the atoms as in C=C. It is important to realize, however, that the two bonds are different: one is a sigma bond, while the other is a pi bond.
::在传统的刘易斯电子-点结构中,双重债券显示为C=C原子之间的双折。 然而,重要的是要认识到这两种债券是不同的:一种是西格玛债券,而另一种是比比债券。Ethyne (C 2 H 2 ) is a linear molecule with a triple bond between the two carbon atoms (see Figure ). The hybridization is therefore sp .
::Ethyne(C2H2)是一种线性分子,在两个碳原子之间有三联(见图)。Ethyne structure.
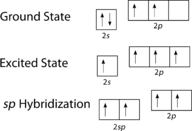
::Ethyne结构。The promotion of an electron in the carbon atom occurs in the same way. However, the hybridization now involves only the 2s orbital and the 2p x orbital, leaving the 2p y and the 2p z orbitals unhybridized.
::碳原子中电子的促销也以同样的方式发生,但现在混合化只涉及轨道2和2px轨道,使2py和2pz轨道没有节制。Hybridization in ethyne.
::乙基苯的混合。The sp hybrid orbitals form a sigma bond between each other as well as sigma bonds to the hydrogen atoms. Both the p y and the p z orbitals on each carbon atom form pi bonds between each other. As with ethene, these side-to-side overlaps are above and below the plane of the molecule. The orientation of the two pi bonds is that they are perpendicular to one another (see Figure ). One pi bond is above and below the line of the molecule as shown, while the other is in front of and behind the page.
::螺旋混合轨道在相互之间形成西格玛联系,并与氢原子形成西格玛联系。 每一个碳原子上的py 和 pz 轨道是彼此的pi 联系。 至于乙烯, 这些侧对侧重叠在分子平面上方和下方。 两个 pi 联系的定位是它们相互垂直(见图 )。 一个 pi 联系在分子线上和下方, 而另一个在页面前和后方。The C 2 H 2 molecule contains a triple bond between the two carbon atoms, one of which is a sigma bond, and two of which are pi bonds.
::C2H2分子含有两个碳原子之间的三重连接,其中一个是西格玛联结,两个是皮联结。In general, single bonds between atoms are always sigma bonds. Double bonds are comprised of one sigma and one pi bond. Triple bonds are comprised of one sigma bond and two pi bonds.
::一般而言,原子之间的单一债券总是西格玛债券,双面债券包括一个西格玛债券和一个比比债券,三面债券包括一个西格玛债券和两个比比债券。Summary
::摘要-
Sigma bonds form between two atoms.
::在两个原子之间形成西格玛联结 -
Pi bonds form from p orbital overlap.
::从轨道重叠形成的皮质保证金形式。
Review
::回顾-
What is the hybridization around each carbon in ethene?
::乙烯中每种碳的混合法是什么? -
What are the two types of bonds in C=C?
::C=C中的两种债券是什么类型? -
What is the shape of the ethene molecule?
::乙烯分子的形状是什么? -
How are the ethyne pi bonds oriented in relation to each other?
::赛恩皮皮债券是如何相互牵线搭桥的?
-
Sigma bonds form between two atoms.