10.9 摩尔多瓦路线图
Section outline
-
How do I get from here to there?
::我怎么从这里到那里?If I want to visit the town of Manteo, North Carolina, out on the coast, I will need a map of how to get there. I may have a printed map or I may download directions from the internet, but I need something to get me going in the right direction. Chemistry road maps serve the same purpose. How do I handle a certain type of calculation? There is a process and a set of directions to help.
::如果我想访问北卡罗来纳州的曼提奥镇,在海岸外,我需要一张如何到达那里的地图。我可能有一张印刷的地图,或者我可以从互联网上下载一些指示,但我需要一些东西才能使我走上正确的方向。化学路线图也有同样的目的。我如何处理某种计算方法?有一个过程和一套方向可以帮助我。Mole Road Map
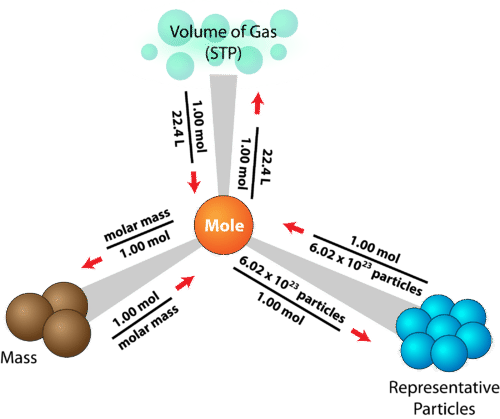
::分子路线图Previously, we saw how the required two steps, with moles as the intermediate . This concept can now be extended to also include volume at STP. The resulting diagram is referred to as a mole road map (see Figure ).
::以前,我们看到了所需的两个步骤,以内鬼为中间点,现在这个概念可以扩大到也包括受威胁人民协会的量。 由此产生的图表被称为内鬼路线图(见图 )。The mole road map shows the conversion factors needed to interconvert between mass, number of particles, and volume of a gas.
::摩尔路线图显示了在气体质量、粒子数量和体积之间相互转换所需的转换系数。The mole is at the center of any calculation involving amount of a substance . The sample problem below is one of many different problems that can be solved using the mole road map.
::内鬼是任何涉及物质数量的计算的中心。下面的抽样问题是许多不同的问题之一,可以通过内鬼路线图加以解决。Sample Problem One: Mole Road Map
::抽样问题一:分子路线图What is the volume of 79.3 g of neon gas at STP?
::受威胁人民协会的79.3克奈恩气体的体积是多少?Step 1: List the known quantities and plan the problem.
::第1步:列出已知数量并规划问题。Known
::已知已知-
Ne = 20.18 g/mol
::Ne=20.18克/摩尔 -
1 mol = 22.4 L
::1毫毫升=22.4升
Unknown
::未知-
volume = ? L
::体积=L
The conversion factors will be grams → moles → gas volume.
::换算系数为克-摩尔-气积。Step 2: Calculate.
::第2步:计算。
::79.3克 Nex1 mol Ne20.18克 Nex22.4 L Ne1 mol Ne=88.0 L NeStep 3: Think about your result.
::步骤3:想想你的结果。The given mass of neon is equal to about 4 moles, resulting in a volume that is about 4 times larger than molar volume .
::给定的纳米质量等于大约4个摩尔,其体积比摩尔体积大约4倍。Summary
::摘要-
An overall process is given for calculations involving moles, grams, and gas volume.
::对涉及摩尔、克和气体体积的计算有一个总体过程。
Review
::回顾-
In the problem above, what is the formula weight of neon?
::在上述问题中,月亮的公式重量是什么? -
What value is at the center of all the calculations?
::所有计算的核心值是什么? -
If we had 79.3 grams of Xe, would we expect a volume that is greater than or less than that obtained with neon?
::如果我们有79.3克的Xe, 我们是否预期其体积会大于或小于用荧光获得的体积?
-
Ne = 20.18 g/mol