11.4 组合反应
章节大纲
-
How useful is a wheel rim?
::轮轮轮轮有多有用?A wheel rim is not very useful by itself. Driving on the rim can damage it and make for a very rough ride. When the rim is combined with a tire, the product can be put on a car and used for a safe and comfortable ride. The two separate items have combined to make something that improves the car ride.
::车轮轮轮圈本身没有多大用处。 驾驶轮轮圈会损坏轮圈,并造成非常艰难的旅程。 当轮圈与轮胎结合时,产品可以放在汽车上,用于安全舒适的旅程。 这两件分开的物品合在一起,可以改善车程。Combination Reactions
::组合反应A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called synthesis reactions. The general form of a combination reaction is:
::组合反应是一种反应,其中两种或多种物质合并成一种单一的新物质。组合反应也可以称为合成反应。组合反应的一般形式是:
::A+BABOne combination reaction is two combining to form a . Solid sodium metal reacts with chlorine to produce solid sodium chloride.
::固态钠金属与氯发生反应,产生固态氯化钠。
::2Na(s)+Cl2(g)%2NaCl(s)Notice that in order to write and balance the equation correctly, it is important to remember the seven elements that exist in nature as diatomic molecules (H 2 , N 2 , O 2 , F 2 , Cl 2 , Br 2 , and I 2 ).
::请注意,为了正确写出和平衡方程,重要的是要记住作为二原子分子(H2、N2、O2、O2、F2、Cl2、Br2和I2)在性质上存在的七个元素。One sort of combination reaction that occurs frequently is the reaction of an element with oxygen to form an oxide. and both react readily with oxygen under most conditions. Magnesium reacts rapidly and dramatically when ignited, combining with oxygen from the air to produce a fine powder of magnesium oxide.
::经常发生的一种组合反应是氧元素与氧形成氧化物的反应,两者在多数条件下都与氧发生随时反应。 镁在点燃时反应迅速和剧烈,与空气中的氧结合产生氧化镁粉末。
::2兆克+O2(g)%2兆克(s)Sulfur reacts with oxygen to form sulfur dioxide.
::硫磺与氧反应形成二氧化硫
::S(s)+O2(g)_SO2(g)When nonmetals react with one another, the product is a molecular compound. Often, the nonmetal reactants can combine in different ratios and produce different products. Sulfur can also combine with oxygen to produce sulfur trioxide.
::当非金属相互反应时,该产品是一种分子化合物,通常非金属反应剂可以以不同的比例结合,产生不同的产品。硫也可以与氧结合,生产三氧化硫。
::2S(s)+3O2(g)%2SO3(g)are capable of adopting multiple positive charges within their . Therefore, most transition metals are capable of forming different products in a combination reaction. Iron reacts with oxygen to form both iron(II) oxide and iron(III) oxide.
::因此,大多数过渡性金属能够在组合反应中形成不同的产品。铁与氧反应形成氧化铁和氧化铁。
::2Fe+O2(g)2FeO4Fe+3O2(g)2Fe2O3
Sample Problem: Combination Reactions
::问题:合并反应Potassium is a very reactive alkali metal that must be stored under oil in order to prevent it from reacting with air. Write the balanced chemical equation for the combination reaction of potassium with oxygen.
::钾是一种反应性很强的碱性金属,必须储存在石油之下,以防止它与空气发生反应。写出钾与氧的混合反应的平衡化学方程式。Step 1: Plan the problem.
::第1步:规划问题。Make sure formulas of all are correct before balancing the equation. Oxygen gas is a diatomic molecule . Potassium oxide is an ionic compound and so its formula is constructed by the crisscross method. Potassium as an becomes K + , while the oxide ion is O 2− .
::在平衡方程之前,确保所有方程式均正确。氧气是一种二原子分子。氧化钾是一种离子化合物,因此其公式是用晶体交叉法构造的。当氧化离子为O2-时,钾变成K+,氧化离子变成O2+。Step 2: Solve.
::步骤2:解决。The skeleton (unbalanced) equation:
::骨骼(不平衡)等式:
::K(s)+O2(g)+K2O(s)The equation is then easily balanced with coefficients .
::然后,这个等式很容易与系数平衡。
::4K(s)+O2(g)%2K2O(s)Step 3: Think about your result.
::步骤3:想想你的结果。Formulas are correct and the resulting combination reaction is balanced.
::公式是正确的,由此产生的组合反应是平衡的。
Combination reactions can also take place when an element reacts with a compound to form a new compound composed of a larger number of atoms. Carbon monoxide reacts with oxygen to form carbon dioxide according to the equation:
::当元素与化合物发生反应形成由更多原子组成的新化合物时,也可以发生混合反应。一氧化碳与氧发生反应,根据等式形成二氧化碳:
::2CO(g)+O2(g)+O2(g)+2CO2(g)Two compounds may also react to from a more complex compound. A very common example is the reactions of oxides with water. Calcium oxide reacts readily with water to produce an aqueous solution of calcium hydroxide.
::一种非常常见的例子是氧化物与水的反应,氧化钙与水随时发生反应,产生氢氧化钙的水溶液。
::CAO(s)+H2O(l)Ca(OH)2(q)Sulfur trioxide gas reacts with water to form sulfuric . This is an unfortunately common reaction that occurs in the atmosphere in some places where oxides of sulfur are present as pollutants. The acid formed in the reaction falls to the ground as acid rain.
::三氧化硫与水发生反应,形成硫化。不幸的是,这在大气中是一种常见的反应,在有些地方,氧化硫作为污染物存在于大气中。 反应中形成的酸随着酸雨落到地上。
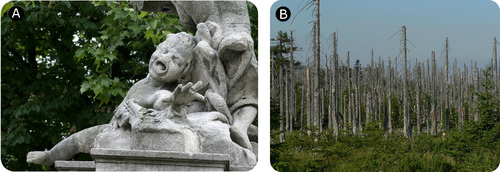
::SO3(g)+H2O(l)+H2O(l)+H2SO4(aq)Acid rain has severe consequences on both natural and man-made objects. Acid rain degrades marble statues like the one on the left (A). The trees in the forest on the right (B) have been killed by acid rain. Summary
::摘要-
Combination reactions occur when two or more substances combine to form a new substance.
::当两种或两种以上物质合在一起形成一种新物质时,就会发生合并反应。
Review
::回顾-
What are combination reactions?
::什么是组合反应? -
Write the product of the following reaction:
::写入下列反应的产物: Mg+H2O -
Is
a combination reaction? Explain your answer.
::CH4+2O2 CO2+2H2O是否是一种组合反应? 请解释您的答复 。
-
Combination reactions occur when two or more substances combine to form a new substance.