13.6 地面紧张局势
章节大纲
-
How is this insect able to stand on water?
::这只昆虫怎么能站在水上?The next time you are by a still body of water, take a close look at what is scooting along on the surface. You may see insects seemingly floating on top of the water. These creatures are known by a variety of names including water skaters, water striders, pond skaters, and other equally descriptive names. They take advantage of a property called surface tension to stay above the water and not sink. The force they exert downward is less than the forces exerted among the water molecules on the surface of the pond, so the insect does not penetrate beneath the surface of the water.
::下次你们在死水边的时候, 仔细观察一下在水面上 爬行的东西。 你可以看到在水面上漂浮的昆虫。 这些生物的名字多种多样, 包括滑水者、 水流者、 池塘滑冰者, 以及其他同样描述性的名字。 它们利用地表紧张状态 留在水面之上, 而不是沉没。 它们向下施加的力量比池塘表面的水分子所施加的力量要小, 所以昆虫不会渗入水面之下 。Surface Tension
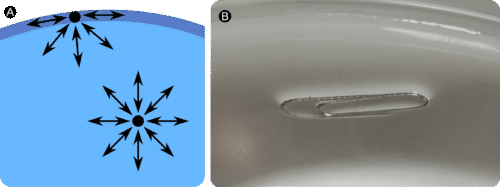
::表面紧张Molecules within a are pulled equally in all directions by intermolecular forces . However, molecules at the surface are pulled downwards and sideways by other liquid molecules, but not upwards away from the surface. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see A in Figure ). The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Liquids that have strong intermolecular forces, like the in water, exhibit the greatest surface tension. Surface tension allows objects that are denser than water, such as the paper clip shown in B in Figure , to nonetheless float on its surface. It is also responsible for the beading up of water droplets on a freshly waxed car because there are no attractions between the polar water molecules and the nonpolar wax.
::分子内部的分子分子在各种方向上都平等地拉动分子。 但是,表面的分子被其他液体分子往下和向侧拉动, 而不是从表面向上拉动。 总体效果是表面分子被拉入液体, 产生一个像胶片一样紧凑的表面(见图A ) 。 液体的表面张力是液体表面弹性力的量度。 具有很强的分子间联力的液体, 如水中, 表现出最大的表面张力。 表面张力使得比水密度更大的物体, 如图B中显示的纸片, 仍然可以漂浮在其表面上。 它还要负责将水滴放在新鲜蜡的汽车上, 因为极地水分子和非极地蜡之间没有吸引力。(A) Molecules at the surface of a liquid are pulled downwards into the liquid, creating a tightened surface. (B) Surface tension allows a paper clip to float on water’s surface.
:A) 液体表面的分子向下拉入液体中,形成一个紧凑的表面。 (B) 表面的紧张使得纸片可以漂浮在水面上。
Other liquids, such as diethyl ether , do not demonstrate strong surface tension interactions. The intermolecular forces for the ether are the relatively weak dipole- dipole interactions that do not draw the molecules together as tightly as would.
::其他液体,如二乙醚,没有表现出强大的表面张力相互作用。 乙醚的分子间系力量是相对薄弱的二极相互作用,它们不会像往常那样紧密地将分子吸引在一起。Science Friday: Candy Corn in Space
::科学星期五:太空糖果玉米Candy corn is a very tasty treat. In this video by Science Friday, astronaut Don Pettit uses Candy Corn to demonstrate the effects of hydrophobic and hydrophilic interactions.
::玉米糖果是一个非常美味的乐谱。在这个由科学星期五拍摄的视频中,宇航员唐·佩蒂特利用玉米糖果展示了疏水和水利相互作用的影响。Summary
::摘要-
The surface tension of a liquid is a measure of the elastic force in the liquid’s surface.
::液体表面的张力是测量液体表面弹性力的尺度。 -
Liquids with strong intermolecular forces have higher surface tensions than liquids with weaker forces.
::内分子力强的液体表面紧张程度高于力量较弱的液体。
Review
::回顾-
Define surface tension.
::确定表面张力。 -
What is responsible for the strong surface tension in water?
::水的地表紧张是何原因? -
Does diethyl ether have a stronger or weaker surface tension than water?
::二乙醚的地表张力是否比水更强或更弱?
-
The surface tension of a liquid is a measure of the elastic force in the liquid’s surface.