13.7 蒸发
章节大纲
-
What is that box on the house's roof?
::房子屋顶上的盒子是什么?On the roof of the house in the picture above is a device known as a “swamp cooler.” This piece of equipment traces its origin back to the ancient Egyptians who hung wet blankets across the doors of their homes. As the warm air passed through the blankets, water would evaporate and cool the air. The royalty went one step further and had servants fan wet cloths over jugs of water to get more evaporation and cooling.
::上面照片中的房子屋顶上有一个称为“湿凉器”的装置。 这一设备可以追溯到古埃及人身上,他们把湿毯子挂在房门对面。 随着温暖的空气从毯子穿透,水会蒸发和冷却空气。 皇室更进一步,让仆人用湿布泡在水壶上,以获得更多的蒸发和冷却。The origin of the term “swamp cooler” is not known – they certainly don’t work in a swamp. Best conditions for cooling include a high temperature (over 80°F) and a low humidity (preferably less than 30%). These coolers work well in desert areas, but don’t provide any cooling in the humid areas of the country.
::冷却的最佳条件包括高温(80摄氏度以上 ) 和低湿度(最好低于30% ) 。 这些冷却器在沙漠地区运作良好,但在该国潮湿地区不提供任何冷却。Evaporation
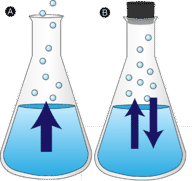
::蒸发A puddle of water left undisturbed eventually disappears. The molecules escape into the phase , becoming water vapor . Vaporization is the process in which a liquid is converted to a gas. Evaporation is the conversion of a liquid to its vapor below the temperature of the liquid. If the water is instead kept in a closed container, the water vapor molecules do not have a chance to escape into the surroundings and so the water level does not change. As some water molecules become vapor, an equal number of water vapor molecules condense back into the liquid state. is the from a gas to a liquid.
::将液体转化为气体的过程。蒸发是将液体转化为气体的过程。蒸发是将液体转化为液体温度以下的蒸发器。如果将水放在封闭容器中,水蒸发分子就没有机会逃入周围,因此水位不会改变。随着一些水分子变成蒸发器,同样数量的水蒸发器分子从气体浓缩到液体状态。Evaporation (A) and condensation (B).
::蒸发(A)和凝聚(B)。In order for a liquid molecule to escape into the gas state, the molecule must have enough to overcome the intermolecular attractive forces in the liquid. Recall that a given liquid sample will have molecules with a wide range of kinetic energies. Liquid molecules that have this certain threshold kinetic energy escape the surface and become vapor. As a result, the liquid molecules that remain now have a lower average kinetic energy. As evaporation occurs, the temperature of the remaining liquid decreases. You have observed the effects of evaporative cooling. On a hot day, the water molecules in your perspiration absorb body and evaporate from the surface of your skin. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body.
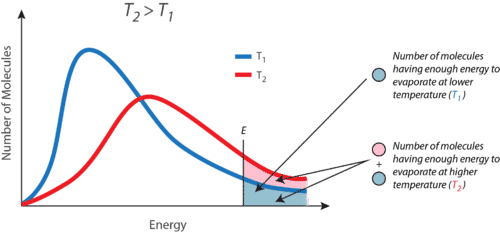
::液分子要逃入气体状态, 分子必须有足够的力量来克服液体中的分子间隙诱导力。 提醒注意, 特定液体样品将拥有具有多种动能的分子。 具有这种临界动能的液体分子会从表面逃出, 变成蒸发体。 结果, 剩下的液体分子现在具有较低的平均动能。 随着蒸发, 其余液体的温度会下降。 您已经看到蒸发式冷却的影响。 在炎热的一天, 你呼吸中的水分子会吸收身体, 从皮肤表面蒸发。 蒸发过程留下剩下的呼吸冷却器, 它会从身体吸收更多的热量。A given liquid will evaporate more quickly when it is heated. This is because the heating process results in a greater fraction of the liquid’s molecules having the necessary kinetic energy to escape the surface of the liquid. Figure shows the kinetic energy distribution of liquid molecules at two temperatures. The numbers of molecules that have the required kinetic energy to evaporate are shown in the shaded area under the curve at the right. The higher temperature liquid (T 2 ) has more molecules that are capable of escaping into the vapor phase than the lower temperature liquid (T 1 ).
::给定的液体在加热时会更快地蒸发。 这是因为加热过程导致液体分子中有更大一部分具有必要的动能以逃离液体表面。 图显示液体分子在两个温度下的动能分布。 具有所需的动能以蒸发的分子数量显示在右边曲线下方的阴暗区域。 高温液体( T2)比低温液体( T1) 有更多的分子可以逃入蒸发阶段。 高温液体( T2) 具有比低温液体( T1) 更多的分子可以逃入蒸发阶段。Kinetic energy distribution curves for a liquid at two temperatures T 1 and T 2 . The shaded area is the molecules with enough kinetic energy to escape the liquid and become vapor.
::T1和T2温度为两度的液体动能分布曲线,阴暗区域是具有足够动能的分子,足以摆脱液体,成为蒸气。Explore how heat and temperature relate to phase change in this simulation:
::在模拟中探索热和温度与阶段变化的关系:Summary
::摘要-
Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid.
::蒸发是指将液体转化为液体沸腾温度以下的蒸气。 -
Condensation is the change of state from a gas to a liquid.
::浓缩是指状态从气体变为液体。 -
As the temperature increases, the rate of evaporation increases.
::随着温度的上升,蒸发率上升。
Review
::回顾-
Define vaporization.
::定义蒸发。 -
Define evaporation.
::定义蒸发。 -
Define condensation.
::定义凝聚。 -
How does temperature affect the rate of evaporation?
::温度如何影响蒸发速度?
-
Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid.