13.20 水状况图
章节大纲
-
How is it possible to create snowballs?
::怎么可能创造雪球呢?You need a special snow to make the best snowballs. This snow needs to be a little wet so the particles will stick together. Dry snow can be tightly pressed and will form snowballs because the higher pressure causes the snowflakes to melt somewhat. However, when you release the pressure, the snow goes back to a more solid form and the flakes no longer stick together.
::您需要特别的雪来制造最好的雪球。 雪需要稍微湿一点, 这样粒子会粘在一起。 干雪可以被紧紧地压住, 并且会形成雪球, 因为高压会使雪花熔化。 但是, 当您释放压力时, 雪会回到更坚固的形态, 片片会不再粘在一起 。Phase Diagram for Water
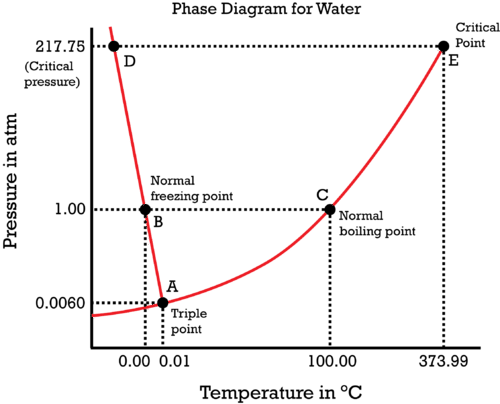
::水的阶段图图Water is a unique substance in many ways. One of these special properties is the fact that solid water (ice) is less dense than water just above the point. The phase diagram for water is shown in Figure .
::在许多方面,水是一种独特的物质,其中一项特殊特性是,固体水(冰)比高于点的水密度要低。Phase diagram for water. Notice one key difference between the general phase diagram and the phase diagram for water. In water’s diagram, the slope of the line between the solid and liquid states is negative rather than positive. The reason is that water is an unusual substance in that its solid state is less dense than the liquid state. Ice floats in liquid water. Therefore, a pressure change has the opposite effect on those two phases. If ice is relatively near its melting point , it can be changed into liquid water by the application of pressure. The water molecules are actually closer together in the liquid phase than they are in the solid phase.
::注意一般相位图和水的相位图之间的一个关键差别。 在水图中, 固体和液体状态之间的线的斜坡是负的,而不是正的。 原因是水是一种不寻常的物质, 因为它的固态比液体状态的密度要低。 冰在液体水中漂浮。 因此, 压力变化对这两个阶段的影响是相反的。 如果冰相对接近其熔点, 就可以通过施压将其改变为液态水。 水分子在液态中实际上比固态更接近。Refer again to water’s phase diagram ( Figure ). Notice point E, labeled the critical point . What does that mean? At 373.99°C, particles of water in the phase are moving very, very rapidly. At any temperature higher than that, the gas phase cannot be made to liquefy, no matter how much pressure is applied to the gas. The critical pressure (P c ) is the pressure that must be applied to the gas at the critical temperature in order to turn it into a liquid. For water, the critical pressure is very high, 217.75 atm. The critical point is the intersection point of the critical temperature and the critical pressure.
::再次参考水相图(图 ) 。 通知点 E 标注了临界点 。 这意味着什么 ? 在 373. 99 °C 时, 水的粒子正在非常非常非常快速地移动。 在任何温度高于此点的情况下, 气相不能被液化, 不论气体承受多少压力 。 关键压力 (Pc) 是必须在临界温度下对气体施压, 才能将其转化为液体 。 对于水, 关键压力非常高, 217. 75 °。 关键点是临界温度和关键压力的交叉点 。Science Friday: Growing Snowflakes in a Bottle
::科学星期五:在瓶子里种植雪花Ever dreamed about making your own snowflakes? It turns out you can, using materials that you can easily obtain. In this video by Science Friday, physicist Ken Libbrecht describes how to build your own snowflake in a plastic bottle.
::曾经梦想过自己制作雪花吗? 事实证明你可以使用很容易获得的材料。 在科学周五的这段视频中,物理学家肯·利布雷赫特(Ken Libbrecht)描述了如何在塑料瓶中建造自己的雪花。Summary
::摘要-
Solid water is less dense than liquid water just above the freezing point.
::固体水的密度低于刚高于冰点的液体水的密度。 -
The critical temperature (T
c
) of a substance is the highest temperature at which the substance can possibly exist as a liquid.
::物质的临界温度(Tc)是该物质可能作为液体存在的最高温度。 -
The critical pressure (P
c
) is the pressure that must be applied to the gas at the critical temperature in order to turn it into a liquid.
::临界压力(Pc)是指必须在临界温度下对气体施加的压力,以便将其转化为液体。 -
The critical point is the intersection point of the critical temperature and the critical pressure.
::关键点是临界温度和临界压力的交叉点。
Review
::回顾-
What happens to solid ice under high pressure near the freezing point?
::在冰点附近的高压下 硬冰会怎么样? -
What is the critical temperature?
::临界温度是多少? -
What is the critical pressure?
::关键压力是什么?
-
Solid water is less dense than liquid water just above the freezing point.