16.11 拟订解决办法
章节大纲
-
How much water do I add?
::我该加多少水?Back in the “good old days” (whenever that really was), many cooks didn’t bother with careful measurements. They “just knew” how much flour to use or how much water to add. Most of us need somewhat more precise ways to measure when we cook. Chemists are very careful when they prepare solutions since the results of their experiments need to be quantitative. “Just knowing” is not accurate enough for scientific purposes.
::回到“好时光 ” ( 不论多么真实 ) , 许多厨师并没有费心仔细地测量。 他们“只知道”需要多少面粉或加多少水。 我们大多数人需要更精确的方法来测量烹饪时间。 化学家在准备解决方案时非常小心,因为他们的实验结果需要量化。 “ 仅仅知道”对于科学目的来说不够准确。Preparing Solutions
::准备解决方案If you are attempting to prepare 1.00 L of a 1.00 molar solution of NaCl, you would obtain 58.44 g of sodium chloride. However you cannot simply add the sodium chloride to 1.00 L of water. After the solute dissolves, the volume of the solution will be slightly greater than a liter because the hydrated sodium and chloride take up space in the solution. Instead, a volumetric flask needs to be used. Volumetric flasks come in a variety of sizes (see image below) and are designed to allow a chemist to prepare a solution only of one specific volume.
::如果您试图准备1.00摩尔溶液的1.00升纳化氢,您将获得58.44克氯化钠。然而,您不能简单地将氯化钠添加到1.00升的水中。溶液溶解后,溶液的体积会略大于一升,因为水化钠和氯化液占了溶液的空间。相反,需要使用一个体积的瓶子。体积的方形不同(见下图),设计时允许化学家只准备一个特定体积的溶液。In other words, you cannot use a 1-liter volumetric flask to make 500 ml of a solution. It can only be used to prepare 1 liter of a solution. The steps to follow when preparing a solution with a 1-liter volumetric flask are outlined below and shown in Figure .
::换句话说,您不能使用一升体积的瓶子来制造500毫升的溶液。它只能用来制造一升体积的溶液。在用一升体积的瓶子准备一种溶液时要遵循的步骤概述如下,并在图中显示。-
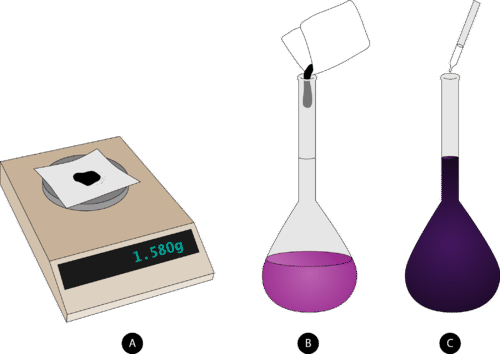
The appropriate mass of solute is weighed out and added to a volumetric flask that has been about half-filled with distilled water.
::溶液的适当质量被权衡出来,并加到一个体积瓶中,该瓶中大约半装有蒸馏水。 -
The solution is swirled until all of the solute dissolves.
::溶液在溶液溶解之前会旋转。 -
More distilled water is carefully added up to the line etched on the neck of the flask.
::蒸馏水被小心地加到 瓶子颈上的缝合线上。 -
The flask is capped and inverted several times to completely mix.
::酒瓶被封住,多次倒转以完全混合。
Steps to follow in preparing a solution of known molarity: (A) weigh out the correct mass of solute, (B) dissolve into the solvent in a volumetric flask, and (C) add solvent to the fill line on the flask and mix. Summary
::摘要-
Steps for the preparation of a solution are outlined.
::报告概述了拟订解决办法的步骤。
Review
::回顾-
Why do you add the solute to the flask only partially filled with solvent?
::为什么把溶液加到酒瓶里 只能部分装满溶剂? -
How much solvent do you add at the end?
::你最后加多少溶剂? -
Why do you invert the flask a few times after diluting to the mark?
::你为什么在稀释到标记后 倒倒酒瓶几次?
Explore More
::探索更多Use the resource below to answer the questions that follow.
::利用以下资源回答以下问题。-
What is a solution?
::什么是解决办法? -
What is the solute?
::什么是溶液? -
What is the solvent?
::溶剂是什么? -
What safety precautions need to be taken?
::需要采取什么安全防范措施?
-
The appropriate mass of solute is weighed out and added to a volumetric flask that has been about half-filled with distilled water.