17.3 异热反应
章节大纲
-
This mushroom cloud was produced in a 1953 nuclear bomb test in Nevada. There’s no doubt that the explosion gave off a huge amount of energy . Although not as impressive as nuclear reactions, many also give off energy. These reactions are called exothermic reactions.
::这一蘑菇云是1953年内华达核炸弹试验中产生的。 毫无疑问,爆炸释放了大量能量。 虽然没有核反应那么令人印象深刻,但许多人也释放了能量。 这些反应被称为异热反应。What Is An Exothermic Reaction?
::什么是异热反应?All chemical reactions involve energy. Energy is used to break bonds in reactants , and energy is released when new bonds form in products. In some chemical reactions, called endothermic reactions, less energy is released when new bonds form in the products than is needed to break bonds in the reactants. The opposite is true of exothermic reactions. In an exothermic reaction , it takes less energy to break bonds in the reactants than is released when new bonds form in the products.
::所有化学反应都涉及能源。能源被用来打破反应器的联结,当产品出现新的联结时,能源就会释放出来。 在一些化学反应中,称为异温反应,当产品出现新的联结时,能量就会减少,而当产品出现新的联结时,能量就会减少。 异常反应的情况正好相反。 在异温反应中,打破反应器的联结所需的能量比产品出现新联结时要少。Energy Change in Exothermic Reactions
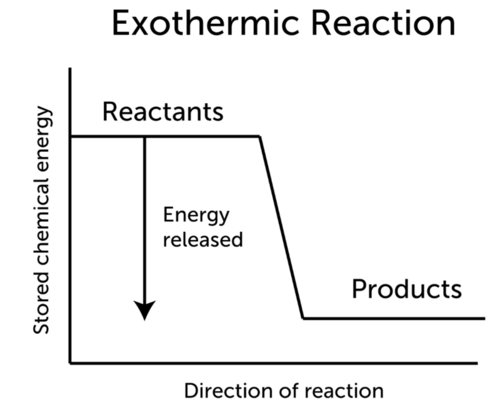
::热热反应中的能源变化The word exothermic means “releasing .” Energy, often in the form of heat, is released as an exothermic reaction proceeds. This is illustrated in the Figure . The general equation for an exothermic reaction is:
::" 异温 " 一词是指 " 释放 " 。 能源,通常以热的形式出现,作为异温反应过程释放出来。-
-
- Reactants → Products + Energy
-
Note: ΔH represents the change in energy.
::注:#H表示能源的变化。If the energy produced in an exothermic reaction is released as heat, it results in a rise in temperature . As a result, the products are likely to be warmer than the reactants.
::如果过热反应产生的能量随着热释放,导致温度上升,因此,这些产品可能比反应物更暖和。Q: You turn on the hot water faucet, and hot water pours out. How does the water get hot? Do you think that an exothermic reaction might be involved?
::问:你打开热水龙头,然后把热水倒出来。水是如何变热的?你认为可能涉及过热反应吗?A: A hot water heater increases the temperature of water in most homes. Many hot water heaters burn a fuel such as natural . The burning fuel causes the water to get hot because combustion is an exothermic reaction.
::A:热热热水加热器提高了大多数家庭的水温,许多热热热水加热器燃烧天然等燃料。燃烧燃料导致水热,因为燃烧是一种异温反应。Combustion as an Exothermic Reaction
::燃烧作为异热反应All combustion reactions are exothermic reactions. During a , a substance burns as it combines with oxygen. When substances burn, they usually give off energy as heat and light. Look at the big bonfire in the Figure . The combustion of wood is an exothermic reaction that releases a lot of energy as heat and light. You can see the light energy the fire is giving off. If you were standing near the fire, you would also feel its heat.
::所有燃烧反应都是过热反应。 在 a 中, 一种物质与氧结合而燃烧。 当物质燃烧时, 它们通常会释放出热和光等能量。 看看图中的大火。 木柴的燃烧是一种释放出大量热和光等能量的过热反应。 您可以看到火所释放的光能。 如果您站在火附近, 你也会感觉到它的热。Summary
::摘要-
An exothermic reaction is a chemical reaction in which less energy is needed to break bonds in the reactants than is released when new bonds form in the products.
::异热反应是一种化学反应,在这种反应中,与产品中新债券形成时所释放的能量相比,在反应器中打破联系所需的能量较少。 -
During an exothermic reaction, energy is constantly given off, often in the form of heat.
::在异热反应期间,能量经常被释放出来,通常以热的形式出现。 -
All combustion reactions are exothermic reactions. During combustion, a substance burns as it combines with oxygen, releasing energy in the form of heat and light.
::所有燃烧反应都是过热反应。燃烧期间,一种物质与氧合在一起燃烧,以热和光的形式释放能量。
Review
::回顾-
What is an exothermic reaction?
::什么是异热反应? -
Why are the products of an exothermic reaction likely to be warmer than the reactants?
::为什么过热反应的产物可能比反应者更温暖? -
Describe an example of an exothermic reaction.
::描述一下过热反应的例子。
Resources
::资源 -