17.8 百虫
章节大纲
-
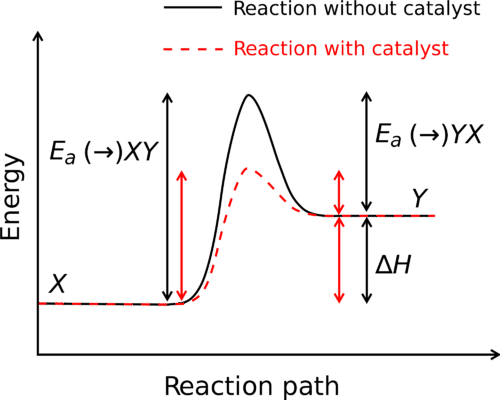
Does the catalyst affect enthalpy?
::催化剂会影响吗?The factors influencing a reaction are complicated and varied. Since a affects , we might assume it would have some sort of impact on enthalpy, but it does not. The change in enthalpy of a reaction depends solely on the chemical compositions of the reactants and products, not on the path taken to get from one to the other.
::影响反应的因素是复杂和多样的。 由于影响,我们可以假设它会对蚂蚁产生某种影响,但不会。 反应的的改变完全取决于反应物和产品的化学成分,而不是从一个到另一个的路径。Enthalpy
::酶changes in are most often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. In that case, the system is at a constant pressure . Enthalpy is the heat content of a system at constant pressure. Chemists routinely measure changes in enthalpy of chemical systems as reactants are converted into products. The heat that is absorbed or released by a reaction at constant pressure is the same as the enthalpy change, and is given the symbol . Unless otherwise specified, all reactions in this material are assumed to take place at constant pressure.
::在反应系统对大气开放的条件下,实验室中测量的变化最经常发生;在这种情况下,系统处于恒定压力下;Enthalpy(H)是系统常压下的热含量;化学家在反应器转换成产品时,经常测量化学系统蚂蚁的含量变化;在不断压力下,反应吸收或释放的热与蚂蚁的变异相同,并标有ZH。除非另有说明,否则假定该材料中的所有反应都在持续压力下发生。The change in enthalpy of a reaction is a measure of the differences in enthalpy of the reactants and products. The enthalpy of a system is determined by the energies needed to break chemical bonds and the energies needed to form chemical bonds. Energy needs to be put into the system in order to break chemical bonds – they do not come apart spontaneously in most cases. Bond formation to produce products will involve release of energy. The change in enthalpy shows the trade-offs made in these two processes. Does it take more energy to break bonds that that needed to form bonds? If so, the reaction is endothermic and the enthalpy change is positive. If more energy is produced in bond formation than that needed for bond breaking, the reaction is exothermic and the enthalpy is negative.
::反应的的改变是反应物和产品的差别的衡量尺度。一个系统的是由打破化学债券所需的能量和形成化学债券所需的能量决定的。能源需要放入这个系统,以便打破化学债券 — — 在多数情况下,它们不会自发解体。生产产品的债券形成将涉及能源的释放。蚂蚁的变化显示了这两个过程的权衡。是否需要更多能量来打破需要形成债券的债券?如果是这样,反应是最终的,而蚂蚁的变化是积极的。 如果债券形成产生的能量比债券破裂所需要的更多,反应是异热的,而蚂蚁是负的。Several factors influence the enthalpy of a system. Enthalpy is an extensive property , determined in part by the amount of material we work with. The state of reactants and products (solid, , or gas) influences the enthalpy value for a system. The direction of the reaction affects the enthalpy value. A reaction that takes place in the opposite direction has the same numerical enthalpy value, but the opposite sign.
::有几个因素影响一个系统的。 酶是一种广泛的属性, 部分取决于我们与之合作的材料的数量。 反应器和产品( 固体、 或气体) 的状况会影响一个系统的值。 反应的方向会影响值。 反方向发生的反应具有相同数字的值, 但相反的符号 。Summary
::摘要-
Enthalpy is related to the heat of a reaction.
::酶与反应的热量有关 -
Factors influencing entropy are described.
::报告介绍了影响酶的因素。
Review
::回顾-
What is enthalpy?
::什么是? -
What is an extensive property?
::什么是大宗财产? -
Do the states of reactants and products influence enthalpy values?
::反应物和产品的状况是否影响过量物值?
-
Enthalpy is related to the heat of a reaction.