18.8 利率法和利率常数
章节大纲
-
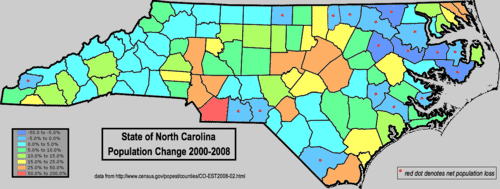
What are the migration patterns of the current population?
::目前人口的移徙模式是什么?Where are people moving from and where are they moving to? How fast is the population changing in different areas? These are important questions for people involved in deciding about where to build schools or hospitals or where to open new businesses. If an area is growing rapidly, action needs to be taken soon to accommodate the growth. How fast the growth is will determine how many schools to build. Rates of change affect a lot of decisions.
::人们从何而来,向何而来?不同领域人口变化的速度有多快?这些对于参与决定在哪里建造学校或医院或在哪里开办新企业的人来说都是重要问题。如果一个地区正在迅速增长,需要尽快采取行动来适应增长。增长的速度将决定多少学校要建设。变化速度影响到许多决定。Rate Law and Specific Rate Constant
::法律利率和具体利率常数Consider a simple in which reactant is converted into product according to the equation below.
::将反应器A按照以下方程式转换成产品B的简单方法加以考虑。
::ABThe rate of reaction is given by the change in of as a function of time. The rate of disappearance of is also proportional to the concentration of .
::A因时间因素的变化给出了反应率,A的失踪率也与A的集中度成正比。
::~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~Since the rate of a reaction generally depends upon collision frequency, it stands to reason that as the concentration of increases, the rate of reaction increases. Likewise, as the concentration of decreases, the rate of reaction decreases. The expression for the rate of the reaction can be shown as follows:
::由于反应率一般取决于碰撞频率,因此有理由认为,随着A的集中度增加,反应率增加。同样,随着A的集中度减少,反应率下降。
::率 [A]orrate=k[A]The proportionality between the rate and becomes an equal sign by the insertion of a constant . A rate law is an expression showing the relationship of the to the concentrations of each reactant . The specific rate constant is the proportionality constant relating the rate of the reaction to the concentrations of reactants. The rate law and the specific rate constant for any chemical reaction must be determined experimentally. The value of the rate constant is temperature dependent. A large value of the rate constant means that the reaction is relatively fast, while a small value of the rate constant means that the reaction is relatively slow.
::通过插入一个常数(k),比例法是一种表达方式,表明每个反应器与浓度之间的关系。具体比率常数(k)是反应率与反应器浓度的相称性常数。必须实验性地确定任何化学反应的比例法和具体比率常数。比率常数的值取决于温度。比率常数的较大值意味着反应相对快,而比率常数的较小值意味着反应相对慢。Summary
::摘要-
The rate law and specific rate constant are defined.
::规定了费率法和具体费率常数。
Review
::回顾-
What is a rate law?
::什么是利率法? -
What is a specific rate constant?
::具体费率的常数是多少? -
How are these parameters determined?
::这些参数是如何确定的?
-
The rate law and specific rate constant are defined.