19.14 预测降水量
章节大纲
-
What do you see?
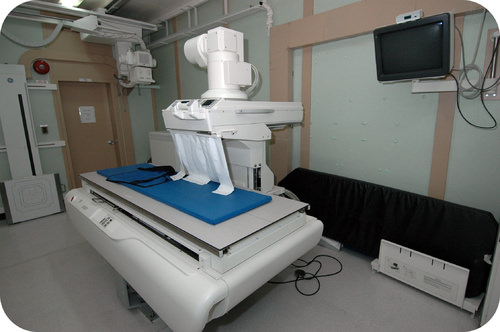
::你看到什么了吗?The invention of the X-ray machine had radically improved medical diagnosis and treatment. For the first time, it was possible to see inside a person’s body to detect broken bones, tumors, obstructions, and other types of problems. Barium sulfate is often used to examine patients with problems of the esophagus, stomach, and intestines. This insoluble coats the inside of the tissues and absorbs X-rays, allowing a clear picture of the interior structure of these organs.
::X光机的发明极大地改善了医疗诊断和治疗。 第一次,在人体内部可以发现骨折、肿瘤、阻塞和其他类型的问题。 血浆硫酸盐经常被用来检查食道、胃和肠道问题患者。 这种无法解脱的外皮在组织内部可以吸收X光,可以清晰地了解这些器官的内部结构。Predicting Precipitates
::预测降水量Knowledge of K s p values will allow you to be able to predict whether or not a precipitate will form when two solutions are mixed together. For example, suppose that a known solution of barium chloride is mixed with a known solution of sodium sulfate. Barium sulfate is a mostly insoluble compound and so could potentially precipitate from the . However, it is first necessary to calculate the product , [Ba 2+ ][SO 4 2− ] for the solution. If the value of the ion product is less than the value of the K s p , then the solution will remain unsaturated. No precipitate will form because the are not high enough to begin the precipitation process. If the value of the ion product is greater than the value of the K s p , then a precipitate will form. The formation of the precipitate lowers the concentration of each of the ions until the ion product is exactly equal to the K s p , at which point precipitation ceases.
::Ksp 值的知识将使您能够预测当两个解决方案混合在一起时, 加速剂是否会形成。 例如, 假设已知的氯化溶液与已知的硫酸钠溶液混在一起。 硫酸主要是溶解的化合物, 因而有可能从溶解中解脱出来。 但是, 首先必须计算溶解的产物, [Ba2+][SO42-] 。 如果离子产品的价值低于 Ksp 的值, 那么溶液将保持不饱和状态 。 因为离子产品的值不足以开始降水过程, 没有沉淀溶液会形成。 如果离子产品的值大于 Ksp 的值, 那么会形成一个沉淀物。 加速剂的形成会降低每种离子的浓度, 直到离子产品与 Ksp 完全相等, 到点降水停止为止 。Barium sulfate is used as a component of white pigment for paints and as an agent in certain x-ray imaging processes.
::硫酸血浆用作油漆白颜料的一部分,并用作某些X射线成像过程的剂。Sample Problem: Predicting Precipitates
::问题:预测降水量Will a precipitate of barium sulfate form when 10.0 mL of 0.0050 M BaCl 2 is mixed with 20.0 mL of 0.0020 M Na 2 SO 4 ?
::当10.0毫升(0.0050 M BaCl2)与20.0毫升(0.0020 M Na2SO4)混合时,硫酸铵会变成硫酸铵吗?Step 1: List the known quantities and plan the problem .
::第1步:列出已知数量并规划问题。Known
::已知已知-
concentration of BaCl
2
= 0.0050 M
::BaCl2 = 0.0050米 -
volume
of BaCl
2
= 10.0 mL
::BaCl2 = 10.0毫升 -
concentration of Na
2
SO
4
= 0.0020 M
::纳2SO4 = 0.0020 M -
volume of Na
2
SO
4
= 20.0 mL
::Na2SO4 = 20.0毫升 -
K
s
p
of BaSO
4
= 1.1 × 10
-10
::BaSO4的Ksp = 1.1× 10-10
Unknown
::未知-
ion product [Ba
2+
][SO
4
2-
]
::离子产品 [BA2+][SO42-] -
if a precipitate forms
::如果急迫地形成,
The concentration and volume of each solution that is mixed together must be used to calculate the [Ba 2+ ] and the [SO 4 2− ]. Each individual solution is diluted when they are mixed together. The ion product is calculated and compared to the K s p to determine if a precipitate forms.
::每种混合溶液的集中度和体积必须用来计算[Ba2+]和[SO42-]。当每种溶液混合在一起时,每种溶液都会被稀释。离子产物是计算出来的,并与Ksp比较,以确定是否是急转式。Step 2: Solve .
::步骤2:解决。The moles of each ion from the original solutions are calculated by multiplying the by the volume in liters .
::原始溶液中的每离子的摩尔是用乘积乘以升数计算的。mol Ba 2 + = 0.0050 M × 0.010 L = 5.0 × 10 − 5 mol Ba 2 + mol SO 2 − 4 = 0.0020 M × 0.020 L = 4.0 × 10 − 5 mol SO 2 − 4
::0.0050 mx0.010 L=5.0x10-5 mol Ba2+摩尔SO2-4=0.0020 Mx0.020 L=4.0x10-5 mol SO2-4-4The concentration of each ion after is then calculated by dividing the moles by the final solution volume of 0.030 L.
::然后计算每离子的浓度,计算方法是将摩尔除以0.030升的最后溶液体积。[ Ba 2 + ] = 5.0 × 10 − 5 mol 0.030 L = 1.7 × 10 − 3 M [ SO 2 − 4 ] = 4.0 × 10 − 5 mol 0.030 L = 1.3 × 10 − 3 M
::[BA2+]=5.0x10-5 mol0.030 L=1.7x10-3 M[SO2-4]=4.0x10-5 mol0.030 L=1.3x10-3 MNow the ion product is calculated.
::现在,离子产品是计算出来的。[ Ba 2 + ] [ SO 2 − 4 ] = ( 1.7 × 10 − 3 ) ( 1.3 × 10 − 3 ) = 2.2 × 10 − 6
::[BA2+][SO2-4]=(1.7×10-3)(1.3×10-3)=2.2×10-6Since the ion product is greater than the K s p , a precipitate of barium sulfate will form.
::由于离子产品大于Ksp, 硫酸将形成。Step 3: Think about your result .
::步骤3:想想你的结果。Two are appropriate for the calculated value of the ion product.
::两种适合离子产品计算值。Summary
::摘要-
Calculations are shown which allow the prediction of precipitate formation based on
K
s
p
.
::所显示的计算方法可以预测基于Ksp的沉淀形成。
Review
::回顾-
What would be the equation for the ion product of BaCl
2
?
::BaCl2的离子产物的方程是什么? -
What happens if the ion product is less than the
K
s
p
?
::如果离子产品小于Ksp怎么办? -
Why did we not need to calculate an ion product for NaCl?
::为什么我们不需要为NACl计算离子产品?
-
concentration of BaCl
2
= 0.0050 M