25.7 环球碳氢化合物
章节大纲
-
Going from benzene to balloons
::从苯到气球Although cyclohexane can be isolated from petroleum products, a major source of this chemical is the hydrogenation of benzene. Much of the cyclohexane produced is used to manufacture intermediates for the production of nylon. The nylon balloons pictured above no doubt had their start in a chemical plant where hydrogen and benzene were reacted at high temperatures to form cyclohexane. This cycloalkane then undergoes nitration to begin the process of forming the long strands of nylon that can be made into balloons, ropes, clothing, and many other useful products.
::尽管六氯环己烷可以与石油产品分离,但这种化学品的一个主要来源是苯的氢化,所生产的大部分六氯环己烷用于制造生产尼龙的中间体,上面的尼龙气球无疑是从一个化学工厂开始的,该工厂的氢和苯在高温下反应形成六氯环己烷,而该环烷则经过硝化过程,开始形成长长的尼龙束,可以制成气球、绳子、衣服和许多其他有用的产品。Cyclic Hydrocarbons
::Cyclic 碳氢碳氢化合物A cyclic is a in which the carbon chain joins to itself in a ring. A cycloalkane is a cyclic hydrocarbon in which all of the carbon-carbon bonds are single bonds. Like other alkanes , cycloalkanes are saturated compounds. Cycloalkanes have the general formula of C n H 2n . The simplest cycloalkane is cyclopropane, a three-carbon ring.
::环形是碳链在环形圈中相互连接的环形环形。环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环形环Cyclopropane is the simplest cycloalkane. Its highly strained geometry makes it rather unstable and highly reactive.
::丙丙烷是最简单的环烷,其高度紧张的几何学使其相当不稳定和反应性很强。The structural formulas of cyclic hydrocarbons can be represented in multiple ways, two of which are shown above. Each can be shown as in the structure on the left from Figure . A convenient shorthand is to omit the symbols and only show the shape, as in the triangle on the right. Carbon atoms are understood to be the vertices of the triangle.
::圆性碳氢化合物的结构公式可以以多种方式表示,其中两个方式在上文显示。每个方式可以像图中左侧结构一样显示。方便的速记是省略符号,仅显示形状,如右侧三角形。碳原子被理解为三角形的顶端。The carbon atoms in cycloalkanes are still sp 3 hybridized, with an ideal bond angle of 109.5°. However, an examination of the cyclopropane structure shows that the triangular structure results in a C-C-C bond angle of 60°. This deviation from the ideal angle is called ring strain and makes cyclopropane a fairly unstable and reactive molecule. Ring strain is decreased for cyclobutane, with a bond angle of 90°, but is still significant. Cyclopentane has a bond angle of about 108°C. This minimal ring strain for cyclopentane makes it a more stable .
::环烷中的碳原子仍然被螺旋3混合,其理想联系角为109.5°。然而,对环丙烷结构的检查表明,三角结构导致C-C-C联系角为60°。这种与理想角的偏差被称为环状菌株,使环丙烷成为相当不稳定的活性分子。环丙烷的环丁烷菌株减少,其粘结角为90°,但仍然很重要。环丙烷的粘结角约为108°C。环丙烷的这种最小环状菌株使其更加稳定。Cyclohexane is a six-carbon cycloalkane shown below.
::Cyclohexane是一种六碳环烷,如下所示。
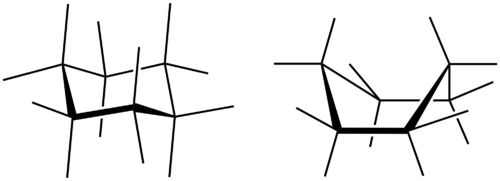
All three of the depictions of cyclohexane are somewhat misleading because the molecule is not planar. In order to reduce the ring strain and attain a bond angle of approximately 109.5°, the molecule is puckered. The puckering of the ring means that every other carbon atom is above and below the plane. Figure shows two possibilities for the puckered cyclohexane molecule. Each of the structures is called a conformation. The conformation on the right is called the boat conformation, while the one on the left is called the chair conformation.
::环氧环已烷的所有三个描述都具有某种误导性,因为分子不是平面分子。为了减少环状菌株并达到大约109.5°的联结角,分子被压住。环的压住意味着所有其他碳原子都在平面上和下方。图显示了被压住的环氧环已烷分子的两种可能性。每个结构都被称为符合。右面的对称称为船对齐,而左面的对齐则称为椅子对齐。Chair (left) and boat (right) conformations for cyclohexane.
::环乙烷的座椅(左)和船(右)符合。While both conformations reduce the ring strain compared to a planar molecule, the chair is preferred. This is because the chair conformation results in fewer repulsive interactions between the hydrogen atoms. However, interconversion readily occurs between the two conformations.
::虽然这两种异质比平板分子减少了环状菌株,但更倾向于采用椅子。这是因为椅子异质导致氢原子之间的反感互动较少,但两种异质之间很容易发生互换。Larger cycloalkanes also exist, but are less common. Cyclic hydrocarbons may also be unsaturated. A cycloalkene is a cyclic hydrocarbon with at least one carbon-carbon double bond. A cycloalkyne is a cyclic hydrocarbon with at least one carbon-carbon triple bond. Shown below are the simplified structural formulas for cyclohexene and cyclooctyne.
::大环烷也存在,但不太常见。 环烷烃也可能不饱和。环烷烃是一种循环碳氢化合物,至少有一个碳碳双联。环烷是一种循环碳氢化合物,至少有一个碳三联。以下为环己烷和环球环球的简化结构公式。
Review
::回顾-
Why is cyclopropane so reactive?
::为什么环丙烷如此被动反应? -
Why is cyclopentane stable?
::为什么环戊烷稳定? -
Name the two forms of cyclohexane.
::列出两种形式的环已烷。
-
Why is cyclopropane so reactive?