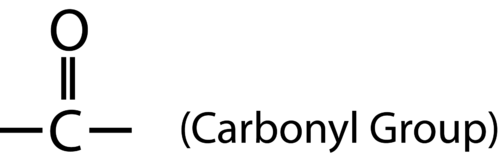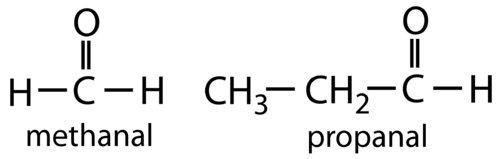25.11 甲和甲
章节大纲
-
Can you resist the smell of a fresh baked cinnamon bun?
::你能抵抗新鲜烤肉桂面包的味道吗?There’s nothing like the smell of a fresh cinnamon roll. The taste is even better. But what causes that delicious taste? This flavoring comes from the bark of a tree (actually, several different kinds of trees). One of the major compounds responsible for the taste and odor of cinnamon is cinnamaldehyde. Cinnamon has been widely used throughout the centuries to treat a number of different disorders. In ancient times, doctors believed it could cure snakebite poisoning, freckles, and the common cold. Today there are several studies being carried out on the health benefits of cinnamon. So, enjoy that cinnamon roll – it just might be good for you.
::没有什么比新鲜肉桂卷闻到的味道更好。 味道更好。 但是什么原因导致这种美味的口味?这种口味来自树皮(事实上是几种不同的树 ) 。 肉桂的味道和味道的主要化合物之一是肉桂的味道和味道之一。 肉桂在几个世纪中被广泛用于治疗多种不同的病症。 在古代,医生认为它可以治愈蛇肉中毒、雀斑和普通寒。 如今,对肉桂的健康好处进行了多项研究。 因此,享受肉桂卷 — — 这对你可能很好。Aldehydes and Ketones
::阿尔德丁和凯顿Aldehydes and ketones are two related categories of organic compounds that both contain the carbonyl group , shown below.
::甲醛和氯酮是两种相关的有机化合物,两者都含有碳基类,如下文所示。The difference between aldehydes and ketones is the placement of the carbonyl group within the molecule. An aldehyde is an organic in which the carbonyl group is attached to a carbon at the end of a carbon chain. A ketone is an organic compound in which the carbonyl group is attached to a carbon atom within the carbon chain. The general formulas for each are shown below.
::藻类和金酮之间的区别在于将碳基类放在分子中。甲醛是一种有机,其中碳基类在碳链的末端附于碳。甲啡是一种有机化合物,其中碳基类在碳链中附于碳原子。每种碳基类的一般公式如下所示。For aldehydes, the R group may be a hydrogen atom or any length carbon chain. Aldehydes are named by finding the longest continuous chain that contains the carbonyl group. Change the –e at the end of the name of the alkane to –al .
::对于藻类而言,R组可以是氢原子或任何长度碳链。 甲酰胺的命名方法是找到含有碳基组的最长的连续链。 将烷名称结尾处的甲苯改为-al。For ketones, R and R’ must be carbon chains, of either the same or different lengths. The steps for naming ketones, followed by two examples, are shown below.
::对于氯酮,R和R必须是相同长度或不同长度的碳链。 命名氯酮的步骤,然后是两个例子,见下文。-
Name the parent compound by finding the longest continuous chain that contains the carbonyl group. Change the
–e
at the end of the name of the alkane to
–one
.
::通过找到包含碳基组的最长连续链来命名母体化合物。 将 alkane 名称末尾的 - e 更改为 - one 。 -
Number the carbon atoms in the chain in a way that the carbonyl group has the lowest possible number.
::以碳基组拥有尽可能最低数量的方式计算链条中的碳原子数量。 -
Add the numerical prefix into the name before the name of the ketone.
::在 Ketone 名称之前添加数字前缀到名称中。 -
Use a hyphen between the number and the name of the ketone.
::在数字和 Ketone 名称之间使用连字符 。
Properties of Aldehydes and Ketones
::Aldehydes 和 Ketones 的属性Aldehydes and ketones can work weak with water through the carbonyl oxygen atom. The lower members of both series (3 carbons or fewer) are soluble in water in all proportions. As the length of the carbon chain increases, water decreases. Similar to , neither aldehydes nor ketones can hydrogen bond with themselves. As a result, their boiling points are generally lower than those of . Unlike alkanes however, aldehydes and ketones are due to the more electronegative oxygen atom. The dipole- dipole interactions are stronger than the dispersion forces present in alkanes. The boiling points of aldehydes and ketones are intermediate between those of alkanes and alcohols. For example, the boiling point of ethane is -89°C, ethanal is 20°C, and ethanol is 78°C.
::碳基氧原子中的低级成员(3个碳或更少)在水中可以溶解。随着碳链长度的增加,水会减少。与碳链长度相似,甲酰胺或氯酮都无法与它们自己结合氢。因此,它们的沸点一般都低于藻类。然而,与藻类不同,甲酰胺和氯酮是由于更多的电阴性氧原子造成的。双极与双极的相互作用比藻类中的分散力强。藻类和氯酮的沸点介于烷和酒精的沸点之间。例如,乙烷的沸点是-89°C,乙烷是20°C,乙醇是78°C。Methanal, commonly known as formaldehyde, was once commonly used as a biological preservative for dead animals. In recent years formaldehyde has been shown to be a carcinogen and so has been replaced for this purpose by safer alternatives. Aldehydes are currently used in the production of resins and plastics. The simplest ketone, propanone, is commonly called acetone. Acetone is a common organic solvent that was once used in most nail polish removers, but has largely been replaced by other solvents.
::美甲胺(俗称甲醛)曾被普遍用作死动物的生物防腐剂,近年来甲醛被证明是一种致癌物,因此被更安全的替代品替代。甲醛目前用于生产树脂和塑料。最简单的甲酮丙酮(丙酮)通常被称为丙酮。丙酮是一种常见的有机溶剂,曾经在大多数指甲油去除器中使用过,但大部分已被其他溶剂替代。Review
::回顾-
What is an aldehyde?
::是什么? -
What is a ketone?
::科酮是什么? -
Can aldehydes or ketones hydrogen bond to themselves?
::藻类或氯酮 氢能和它们自己结合吗?
-
Name the parent compound by finding the longest continuous chain that contains the carbonyl group. Change the
–e
at the end of the name of the alkane to
–one
.