25.18 氧化氧化反应
章节大纲
-
How do we keep food safe?
::我们如何保证食物的安全?Benzoic crystals in polarized light are pictured above. Benzoic acid is widely used as a food preservative, either as the carboxylic acid or as the sodium benzoate salt . This is most effective when added to acidic foods such as fruit juices and soft drinks. The major industrial source of benzoic acid is the partial oxidation of toluene with oxygen. The process is inexpensive and environmentally benign.
::在两极化光线下,苯并二酸被广泛用作食品防腐剂,既可用作箱式酸,也可用作苯并酸钠盐,在添加果汁和软饮料等酸性食品时最为有效,苯并二酸的主要工业来源是含氧甲苯的部分氧化。Oxidation Reactions
::氧化氧化值反应Oxidation can be defined as the addition of oxygen to a molecule or the removal of hydrogen from a molecule. When an alkane is heated in the presence of an appropriate , it can be oxidized to the corresponding alkene in a reaction called a dehydrogenation reaction. Two hydrogen atoms are removed in the process. The alkene can be further oxidized to an alkyne by the removal of two more hydrogen atoms.
::氧化可定义为将氧添加到分子中或将氢从分子中去除。当一种碳烷在适当条件下加热时,在一种称为脱水反应的反应中,可将其氧化为相应的烷。在这一过程中,将两个氢原子去除。通过去除另外两个氢原子,可以将碳烷进一步氧化为烷。
::CH3CH3 H2CH2=CH2 H2CH CHCHThe reactions are reversible, and so an alkyne can be reduced first to an alkene and then to an alkane.
::反应是可逆转的, 所以, 烷可以先降低到烷, 然后降低到烷。
::减少排放量:H2CH2=CH2=H2CH3The alkane is the most reduced form of a , while the alkyne is the most oxidized form.
::亚烷是最弱化的形式,而亚烷是最氧化的形式。Oxidation reactions in organic chemistry often involve the addition of oxygen to a compound, which changes the particular functional group of that compound. The following sequence shows how methane can be oxidized first to methanol, then to methanal, then to methanoic acid, and finally to carbon dioxide.
::有机化学中的氧化反应往往涉及化合物中添加氧,这改变了该化合物的特定功能组。 以下顺序显示甲烷如何首先氧化到甲醇,然后氧化到甲醇,然后氧化到甲基酸,最后氧化到二氧化碳。
::CH4增加氧气CH3OH 增加氧气HH2O 增加氧气HCOH 损失二氧化碳乙烷乙醇甲基甲醚酸Each step in the process is either a gain of oxygen or a loss of hydrogen. Each step also releases energy , which explains why the complete combustion of alkanes to carbon dioxide is an extremely .
::这一过程的每一步要么是增加氧气,要么是失去氢气。 每一步还释放出能量,这解释了为什么烷完全燃烧到二氧化碳中是极其严重的。The oxidation of an can produce either an aldehyde or a ketone . Ethanol can be oxidized in the laboratory through a heating process combined with the addition of an oxidizing agent such as the dichromate , which catalyzes the reaction in an acidic solution . The reaction produces the aldehyde ethanal (acetaldehyde).
::乙醇可以通过加热过程在实验室内氧化,同时添加一种氧化剂,如二甲酸酯,用酸溶液催化反应;该反应产生甲醚乙酰胺(乙酰)。
::CH3CH2OH+Cr2O72-CH3CHHCHH+Cr2O72-CH3CHHHH - CH3CHHCHH_H+Cr2O72 -CH3CHHHHH - CHCHH3CHHH - CHCHCHCHH2OH_HH+Cr2O72 - CH3CHHHHHH - CHCHCHCHH - CHCHCHCHH - CHCHCHH2OH_HH+Cr2O72 - CHCHCHHH - CHCHCHHH - CHCHHHHHH - CHHH+Cr2O72 -CHCHCHHH - CHCHCHCHCHCHCHH - CHCHCHH - CH CHCHH - CHHHH - CHHHHHH - CH CHHHHHH - CH - CH CH CH CHHH - CHH - CH CHHHHHHHHH - CHHHHH CHHHHHHHHH+ CHH+Cr2H - CH CHHHH+Cr2CHCHCHCHCHCHH - CHCHCHCHCHH - CHCHCHCHCHCH CH - CH3CHH - CHCHCHCHCH3CH3CH CHCHCHCHCHCHCHCHCHCHCHCHCHCHCH CHCHCHCHCH CHCHCHCHCHCHCH CH CH CH - CH CH CH - CHH - CH - CH CH - CH CH CH - CH CH - CH CHCHCHHHHHHHHHHHH CH - CHCH CH CH CH CH CH CH - CH - CHCHCHCHCH CH CH CH CH CHCHCHCHCHCH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CHH CH CH CH CH CH CH CH CH CH CH CHWhen the alcohol to be oxidized is a secondary alcohol, the oxidation product is a ketone rather than an aldehyde. The oxidation of the simplest secondary alcohol, 2-propanol, yields propanone.
::当要氧化的酒精是二次酒精时,氧化产物是氯酮,而不是。 最简单的二次酒精(2-丙醇)的氧化产生丙酮。
::CH3CHHHH3+Cr2O72-CH3COCH3Tertiary alcohols cannot be oxidized in this way because the carbon to which the hydroxyl group is attached does not have another hydrogen attached to it.
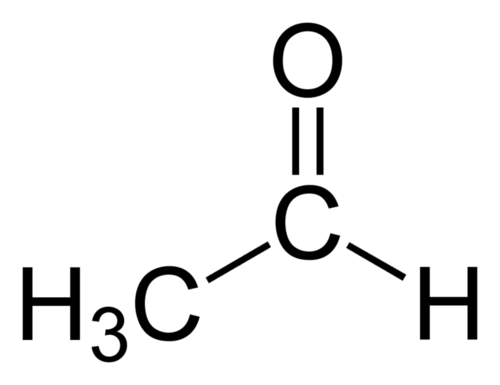
::不能以这种方式氧化三重酒精,因为与氢氧基组相连的碳没有另外的氢。When a primary alcohol is oxidized to an aldehyde, the reaction is difficult to stop because the aldehyde is easily oxidized further to the corresponding carboxylic acid. The oxidation of ethanal produces ethanoic (acetic) acid.
::当一种原生酒精被氧化为aldehyide时,反应难以停止,因为甲醚很容易被氧化至相应的碳酸。 乙醇的氧化会产生代谢(乙酸)酸。
::CHCHH+Cr2O72-CH3COOH CH3HH+Cr2O72-CH3COOH CH3HHH+Cr2O72-CH3COOHEthanol-containing beverages such as wine are susceptible to such oxidation if kept for long periods of time after having been opened and exposed to the air. Wine that has become oxidized will have an unpleasant vinegary taste due to the production of acetic acid.
::含有乙醇的饮料,如葡萄酒,如果在打开并暴露于空气后长时间保持,则容易发生此类氧化;由于生产乙酸,已氧化的葡萄酒将具有令人不愉快的静食性口味。Unlike aldehydes, ketones are resistant to further oxidation because the carbonyl group is in the middle of the carbon chain and so the ketone cannot be converted to a carboxylic acid.
::与藻类不同,氯酮对进一步氧化具有抗药性,因为碳基类处于碳链的中间,因此,氯酮不能转换为箱状酸。Review
::回顾-
What is the product of the oxidation of a primary alcohol?
::初级酒精氧化的产物是什么? -
What type of compound gives a ketone when oxidized?
::当氧化时,哪种化合物会给一个克酮? -
What will produce acetic acid when completely oxidized?
::完全氧化后会产生什么醋酸?
-
What is the product of the oxidation of a primary alcohol?