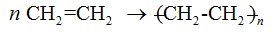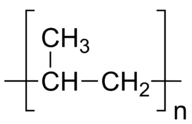25.20 聚合 - 附加聚合物
章节大纲
-
Will it ever go away?
::它会消失吗?We enjoy the benefits of Styrofoam ® containers, but don’t often think about where they end up. Styrofoam ® materials do not break down quickly under exposure to the . When buried in a landfill, styrofoam will remain intact for a long time. The good news is that there is not a lot of this pollutant found in landfills (maybe about 0.5% by weight of the total mass of garbage). There is no good way to recycle Styrofoam ® at present, but someday a creative scientist may come up with one.
::我们享受了泡沫塑料容器的好处,但并不经常想到它们最终会到哪里去。 泡沫泡沫材料不会在暴露于垃圾填埋场下快速崩溃。 当被埋在垃圾填埋场时,泡沫泡沫会长期保持完好无损。 好消息是垃圾填埋场中并未发现很多这种污染物(按垃圾总量的重量计,可能只有0.5 % ) 。 目前没有回收泡沫塑料的好办法,但有朝一日,有创意的科学家可能会找到这种污染物。Polymerization – Addition Polymers
::聚合 - 附加聚合物Polymers are very different from the other kinds of organic molecules that you have seen so far. Whereas other compounds are of relatively low , polymers are giant molecules of very high molar mass. Polymers are the primary components of all sorts of plastics and related compounds. A polymer is a large molecule formed of many smaller molecules covalently bonded in a repeating pattern. The small molecules which make up the polymer are called monomers . Polymers generally form either from an or a condensation reaction .
::聚合物是各种塑料和相关化合物的主要成分。聚合物是一个大型分子,由许多较小的分子组成,以重复形式相互连接。组成聚合物的微小分子称为单分子。聚合物一般来自一种或一种凝聚反应。Addition Polymers
::添加聚合物An addition polymer is a polymer formed by chain addition reactions between monomers that contain a double bond. Molecules of ethene can polymerize with each other under the right conditions to form the polymer called polyethylene.
::添加聚合物是一种聚合物,由含有双重结合的单体之间的连锁反应所形成的聚合物,在形成称为聚乙烯的聚合物的适当条件下,乙烯的分子可以相互聚合。The letter n stands for the number of monomers that are joined in repeated fashion to make the polymer and can have a value in the hundreds or even thousands.
::字母 n 表示重复合并以制造聚合物并具有数百甚至数千个价值的单体数量。Polyethylene synthesis.
::聚乙烯合成。The reactions above show the basic steps to form an addition polymer:
::上述反应表明了形成添加聚合物的基本步骤:-
Initiation - a free radical initiator (X
*
) attacks the carbon-carbon double bond (first step above). The initiator can be something like hydrogen peroxide. This material can easily split to form two species with a free
attached to each: H-O-O-H → 2 H-O•. This free radical attacks a carbon-carbon double bond. One of the pi electrons forms a single bond with the initiator while the other pi electron forms a new free radical on the carbon
.
::启动 - 自由激进启动者( X* ) 攻击碳- 碳双联( 以上第一步 ) 。 启动者可以是过氧化氢。 这种材料可以很容易地分裂成两个物种,每个物种都有一个自由附着的H- O- O- H- 2 H- O- ° 。 这种自由激进打击碳- 双联。 其中一个pi 电子与启动者形成单一的连接, 而另一个pi 电子则形成一个新的碳自由基 。 -
Propagation - the new free radical
interacts with another
alkene
, continuing the process of chain growth (second step above).
::传承----新的自由激进与另一股同业互动,继续连锁增长进程(以上第二步)。 -
Termination occurs whenever two free radicals come in contact with one another (not shown). The two free electrons form a
and the free radical on each molecule no longer exists.
::当两个自由基相互接触(没有显示)时,终止就会发生。 两个自由电子形成一个自由基,而每个分子的自由基不复存在。
Polyethylene can have different properties depending on the length of the polymer chains and on how efficiently they pack together. Some common products made from different forms of polyethylene include plastic bottles, plastic bags, and harder plastic objects such as milk crates.
::根据聚合物链的长度以及聚合物链的包装效率,聚乙烯可以具有不同的特性。 由不同形式的聚乙烯制成的一些常见产品包括塑料瓶、塑料袋和牛奶箱等硬塑料物件。Several other kinds of unsaturated monomers can be polymerized and are components in common household products. Polypropylene is stiffer than polyethylene is in plastic utensils and some other types of containers.
::其他几种不饱和的单体可以聚合,并且是普通家用产品的组成部分,聚丙烯比塑料用具和其他一些类型的容器中的聚乙烯硬。Polypropylene structure.
::聚丙烯结构。Polystyrene is used in insulation and in molded items such as coffee cups.
::聚苯乙烯用于绝缘和模具物品,如咖啡杯。Polystyrene synthesis and structure
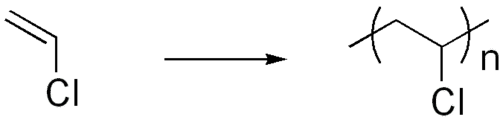
::聚苯乙烯合成和结构Polyvinyl chloride (PVC) is extensively used for plumbing pipes.
::聚氯乙烯(PVC)广泛用于管道管道。Polyvinyl chloride
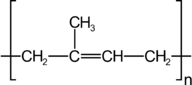
::聚氯乙烯Polyisoprene is a polymer of isoprene and is better known as rubber. It is produced naturally by rubber trees, but several variants have been developed which demonstrate improvements on the properties of natural rubber.
::Polyisoprene是一种异丙烯聚合物,通常称为橡胶,由橡胶树自然产生,但已经开发了几种变异物,表明天然橡胶特性的改善。Polyisoprene
::聚硅烯Review
::回顾-
What is an addition polymer?
::什么是添加聚合物? -
What is the first step in the synthesis of a polymer?
::合成聚合物的第一步是什么? -
What is the final step in the synthesis of a polymer?
::合成聚合物的最后一步是什么?
-
Initiation - a free radical initiator (X
*
) attacks the carbon-carbon double bond (first step above). The initiator can be something like hydrogen peroxide. This material can easily split to form two species with a free
attached to each: H-O-O-H → 2 H-O•. This free radical attacks a carbon-carbon double bond. One of the pi electrons forms a single bond with the initiator while the other pi electron forms a new free radical on the carbon
.