3.2 元素和化合物
章节大纲
-
What Are You Made of?
::你是什么做的吗?If you look at your hand, what do you see? Of course, you see skin, which consists of . But what are skin cells made of? Like all living cells, they are made of matter. In fact, all things are made of matter. Matter is anything that takes up space and has mass. Matter, in turn, is made up of chemical substances. A chemical substance is matter that has a definite composition that is consistent throughout. A chemical substance may be either an element or a compound.
::如果你看着你的手,你看到了什么?当然,你可以看到皮肤,它是由。但是皮肤细胞是由什么组成的?它们和所有活细胞一样,都是物质。事实上,所有的东西都是物质组成的。物质是占地和有质量的东西。物质是化学物质组成的。化学物质是具有一定成分的,并且始终一致。化学物质可以是元素,也可以是化合物。Elements and Atoms
::元素和原子An element is a pure substance. It cannot be broken down into other types of substances. Each element is made up of just one type of atom.
::元素是纯物质,不能细分为其他类型的物质,每种元素由一种原子组成。Structure of an Atom
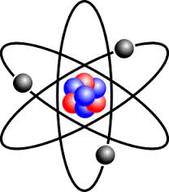
::原子结构An atom is the smallest particle of an element that still has the properties of that element. Every substance is composed of atoms. Atoms are extremely small, typically about a ten-billionth of a meter in diameter. However, atoms do not have well-defined boundaries, as suggested by the atomic model shown .
::原子是一个元素中最小的粒子,该元素仍然具有该元素的特性。每种物质由原子组成。原子非常小,通常直径约为10亿米左右。然而,原子没有如原子模型所示的明确界定的界限。Simple model of an atom
::原子简单模型Every atom is composed of a central area — called the — and one or more subatomic particles called electrons, which move around the nucleus. The nucleus also consists of subatomic particles. It contains one or more protons and typically a similar number of neutrons. The number of protons in the nucleus determines the type of element an atom represents. An atom of hydrogen, for example, contains just one proton. Atoms of the same element may have different numbers of neutrons in the nucleus. Atoms of the same element with the same number of protons — but different numbers of neutrons — are called isotopes.
::每个原子都由一个核心区域组成,称为“原子”和一个或一个以上的亚原子粒子组成,这些子原子粒子被称作电子,在核心周围移动。核心也由亚原子粒子组成。核心包含一个或一个以上的质子,通常包含类似数量的中子。核中的质子数量决定原子所代表的元素类型。例如,氢原子只包含一个质子。同一元素的原子在核核中可能有不同数量的中子。同一元素的原子与质子数量相同,但中子数量不同,则称为同位素。Protons have a positive electric charge and neutrons have no electric charge. Virtually all of an atom's mass is in the protons and neutrons in the nucleus. Electrons surrounding the nucleus have almost no mass, as well as a negative electric charge. If the number of protons and electrons in an atom are equal, then an atom is electrically neutral, because the positive and negative charges cancel each other out. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively, and it is called an ion .
::质子有正电荷, 中子没有电荷。 几乎所有原子的质量都在质子和核中中。 围绕核的电几乎没有质量, 也有负电荷。 如果原子中的质子和电子数量相等, 那么原子在电子上是中和的, 因为正电和负电荷相互抵消。 如果原子的电荷比质子多或少, 那么原子的电荷就分别是负电或正电荷, 被称为离子。The negatively-charged electrons of an atom are attracted to the positively-charged protons in the nucleus by a force called electromagnetic force, for which opposite charges attract. Electromagnetic force between protons in the nucleus causes these subatomic particles to repel each other, because they have the same charge. However, the protons and neutrons in the nucleus are attracted to each other by a different force, called nuclear force, which is usually stronger than the electromagnetic force. Nuclear force repels the positively-charged protons from each other.
::原子的负电荷电子被一个叫做电磁力的力量吸引到核核中的正电荷质子中。 核中的质子之间的电磁力导致这些亚原子粒子相互反射,因为它们具有相同的电荷。 但是,核核中的质子和中子被不同的力量,即通常比电磁力强的核力量相互吸引。 核力量会相互反射正电磁质子。Periodic Table of the Elements
::要点定期表格There are almost 120 known elements. As you can see in the Periodic Table of the Elements shown , the majority of elements are metals. Examples of metals are iron (Fe) and copper (Cu). Metals are shiny and good conductors of electricity and heat. Nonmetal elements are far fewer in number. They include hydrogen (H) and oxygen (O). They lack the properties of metals.
::有近120个已知元素。从显示元素的周期表中可以看出,大部分元素是金属,金属的例子有铁(Fe)和铜(Cu),金属亮亮,电和热的导体良好,非金属元素少得多,包括氢(H)和氧(O),缺乏金属特性。
Periodic Table of the Elements. The periodic table of the elements arranges elements in groups based on their properties. The element most important to life is carbon (C). Find carbon in the table. What type of element is it: metal or nonmetal?
::元素周期表。元素周期表根据元素的特性按组排列元素。对生命最重要的元素是碳(C)。在表格中查找碳。元素的类型是:金属还是非金属?Compounds and Molecules
::化合物和分子A compound is a unique substance that consists of two or more elements combined in fixed proportions. This means that the composition of a compound is always the same. The smallest particle of most compounds in living things is called a molecule.
::化合物是一种独特的物质,由两个或两个以上的元素组成,按固定比例组合。这意味着化合物的构成总是相同的。在生物物质中,大多数化合物中最小的粒子被称为分子。Consider as an example. A molecule of water always contains one atom of oxygen and two atoms of hydrogen. The composition of water is expressed by the chemical formula H 2 O. A model of a water molecule is shown in the figure .
::以水分子为例。水分子总是含有一个氧原子和两个氢原子。水的构成用化学公式H2O表示。一个水分子模型在图中显示。
Water Molecule. A water molecule always has this composition — one atom of oxygen and two atoms of hydrogen.
::水分子总是有这种成分——一个氧原子和两个氢原子。What causes the atoms of a water molecule to “stick” together? The answer is chemical bonds . A chemical bond is a force that holds together the atoms of molecules. Bonds in molecules involve the sharing of electrons among atoms. New chemical bonds form when substances react with one another. A chemical reaction is a process that changes some chemical substances into others. A chemical reaction is needed to form a compound, and another chemical reaction is needed to separate the substances in that compound.
::导致水分子原子“粘合”的原因何在?答案是化学联系。化学联系是一种将分子原子聚集在一起的力量。分子的粘合涉及原子之间的电子共享。当物质相互反应时,新的化学联系形式是新的化学联系形式。化学反应是一种将某些化学物质转化为其他化学物质的过程。需要化学反应来形成化合物,需要另一种化学反应来分离该化合物中的物质。Summary
::摘要-
All matter consists of chemical substances. A chemical substance has a definite composition
which is consistent
throughout. A chemical substance may be either an element or a compound.
::所有物质均由化学物质组成,化学物质具有一定的成分,其成分始终一致,化学物质既可以是元素,也可以是化合物。 -
An element is a pure substance that cannot be broken down into other types of substances.
::元素是不能细分为其他类型物质的纯物质。 -
An atom is the smallest particle of an element that still has the properties of that element. Atoms, in turn, are composed of subatomic particles, including negative electrons, positive protons, and neutral neutrons. The number of protons in an atom determines the element it represents.
::原子是一个元素的最小粒子,该元素仍然具有该元素的特性。原子反过来由亚原子粒子组成,包括负电子、正质子和中中中中子。原子中的质子数量决定其所代表的元素。 -
Atoms have equal numbers of electrons and protons, so they have no charge. Ions are atoms that have lost or gained electrons,
and as a result
have either a positive or negative charge. Atoms with the same number of protons — but different numbers of neutrons — are called isotopes.
::原子拥有相同数量的电子和质子,因此它们没有电荷。离子是已经丢失或获得电子的原子,因此具有正负电荷或负电荷。质子数量相同的原子——但中子数量不同的原子——被称为同位素。 -
There are almost 120 known elements. The majority of elements are metals. A smaller number are nonmetals. The latter include carbon, hydrogen, and oxygen.
::有近120种已知元素,大多数元素是金属,较少的元素是非金属,后者包括碳、氢和氧。 -
A compound is a substance that consists of two or more elements in a unique composition. The smallest particle of a compound is called a molecule. Chemical bonds hold together the atoms of molecules. Compounds can form only in chemical reactions, and they can break down only in other chemical reactions.
::化合物是一种由两个或两个以上元素组成的物质,构成一种独特的成分。一种化合物的最小粒子称为分子。化学联系将分子原子聚集在一起。化合物只能形成化学反应,只能分解到其他化学反应中。
Review
::回顾1. What is an element? Give three examples.
::1. 什么是要素?举三个例子。2. Define compound. Explain how compounds form.
::2. 定义化合物,解释化合物的形态。3. Compare and contrast atoms and molecules.
::3. 比较和对比原子和分子。4. The compound called water can be broken down into its constituent elements by applying an electric current to it. What ratio of elements is produced in this process?
::4. 使用电流可以将称为水的化合物分解成其组成成分,在这一过程中产生何种比例的元素?5. Relate ions and isotopes to elements and atoms.
::5. 将离子和同位素与元素和原子相联系。6. What is the most important element to life?
::6. 生命最重要的要素是什么?7. Iron oxide is often known as rust — the reddish substance you might find on corroded metal. The chemical formula for this type of iron oxide is Fe 2 O 3 . Answer the following questions about iron oxide and briefly explain each answer.
::7. 氧化铁通常被称为生锈——在腐蚀金属上可能发现的红色物质,此类氧化铁的化学公式是Fe2O3。 回答以下关于氧化铁的问题,并简要解释每个答案。a. Is iron oxide an element or a compound?
::a. 氧化铁是元素还是化合物?b. Would one particle of iron oxide be considered a molecule or an atom?
::b. 一个氧化铁粒子是否被视为分子或原子?c. Describe the relative proportion of atoms in iron oxide.
::c. 描述氧化铁中原子的相对比例。d. What causes the Fe and O to stick together in iron oxide?
:d) 是什么原因导致Fe和O聚在一起使用氧化铁?
e. Is iron oxide made of metal atoms, metalloid atoms, nonmetal atoms, or a combination of any of these?
::e. 氧化铁是由金属原子、金属类原子、金属类原子、非金属类原子或任何此类原子的组合制成的吗?8. 14 C is an isotope of carbon used in the radiocarbon dating of organic material. The most common isotope of carbon is 12 C. Do you think 14 C and 12 C have different numbers of neutrons or protons? Explain your answer.
::8.C 14C 是用于有机材料放射性碳代号的碳同位素,最常见的碳同位素是12C。 你认为14C和12C的中子或质子数量不同吗?请解释您的答复。9. Explain why ions have a positive or negative charge.
::9. 解释为什么离子有正或负的收费。10. Name the three subatomic particles described in this section.
::10. 请列出本节描述的三个亚原子粒子。Explore More
::探索更多Watch the video below to learn more about the size of an atom.
::观看下面的视频,以了解更多关于原子大小的知识。Watch the video below to learn about four newly discovered elements.
::看下面的录像,了解四个新发现的元素。 -
All matter consists of chemical substances. A chemical substance has a definite composition
which is consistent
throughout. A chemical substance may be either an element or a compound.