9.4 量度测量
章节大纲
-
Though not particularly beautiful machines, calorimeters are incredibly useful ones. They are used to determine the calories (food energy) in food, as well as the average yield from burning various grades of coal and oil. The price of coal is often dependent on the heat yield from samples burned in a calorimeter.
::热量计虽然不是特别漂亮的机器,但却非常有用。 它们被用来确定食物中的热量(食品能源 ) , 以及燃烧不同等级煤炭和石油的平均产量。 煤炭的价格往往取决于燃烧在热量计中的样品的热量。Calorimetry
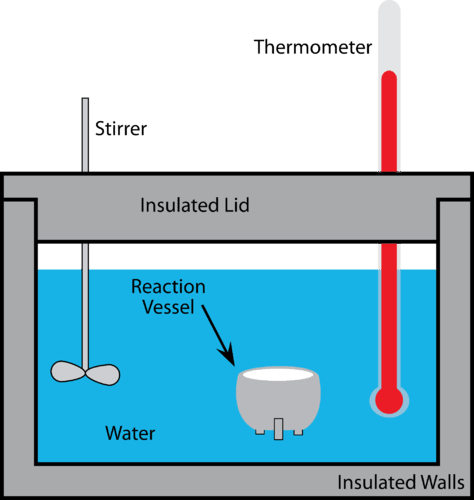
::量度测量A calorimeter is a device used to measure changes in or . More specifically, it measures calories. A calorie is the amount of required to raise one gram of water by one degree Celsius. As such, the calorimeter measures the change of a known amount of water. If a reaction is carried out in the reaction vessel, or if a measured mass of heated substance is placed in the water of the calorimeter, the change in the water temperature allows us to calculate the change in thermal energy.
::热量计是一种测量热量变化的装置,更具体地说,它用来测量热量。热量是将一克水提高一摄氏度所需的量。因此,热量计测量已知水量的变化。如果反应容器中发生反应,或者将测量的加热物质质量置于热量计的水中,水温的变化使我们能够计算热能的变化。The function of the calorimeter depends on the in a closed, isolated system. Calorimeters are carefully insulated so that heat transfer in or out is negligible. This allows scientists to assume the total amount of energy in the system ( ) will remain constant and can be used to derive the following equation.
::热量计的功能取决于封闭的、孤立的系统中的功能。 热量计经过仔细隔绝, 从而可以忽略不计地进行热传导。 这样科学家就可以假设系统中的能量总量( Q) 将保持不变, 并可用于得出以下方程式 。
::m1c1T1m2c2T2Consider the following examples.
::考虑以下例子。Example 1
::例1A 0.500 kg sample of water in a calorimeter is at 15.0ºC. A 0.0400 kg block of zinc at 115.0ºC is placed in the water. The of zinc is 388 J/kg•ºC. Find the final temperature of the system.
::在卡路里计中,0.500公斤水样本为15.0°C。 A0.0400公斤锌块,在115.0°C时放置在水中,锌为388 J/kg °C。寻找系统的最后温度。The heat lost by the block of zinc will equal the heat gain by the water in the calorimeter. In order to set heat gain mathematically equal to heat loss, either one of the terms must be made negative or the temperature change must be reversed. You should also note that the final temperature of the water and the block of zinc will be the same when equilibrium is reached.
::锌块流失的热量等于热量计中水的增热量,为了从数学上确定热增速等于热损耗,必须使其中一个条件为负值,或必须扭转温度变化。你还应注意,当达到平衡时,水和锌块的最后温度将与最后温度相同。
::mWW(t2-t1)W=mZncZn(t1-t2)Zn(0.500千克)(4180 J/kgC)(x-15.0C)=(0.0400千克)(388 J/kgC)(115.0C-x)2090 x-31350=17855-152.52 x2105.52 x=33135x=15.7°C)Example 2
::例2A 100. g block of aluminum at 100.0ºC is placed in 100. g of water at 10.0ºC. The final temperature of the mixture is 25.0ºC. What is the specific heat of the aluminum as determined by the experiment?
::100.0°C时100克铝块放在100克的水中,10.0°C时为10.0克。 混合物的最后温度为25.0°C。 试验确定铝的具体热量是多少?
::mWCW(t2-2-t1)W=mALcAl(t1-t2)AL(0.100千克)(4180 J/kgC)(25.0C-10.0C)=(0.100千克)(x)(100.0C-25.0C)6270=7.50x=836J/kg}CUse the PLIX Interactive below to further explore how a calorimeter can be used by scientists to observe the temperature change that occurs in a reaction:
::利用下面的PLIX互动来进一步探索科学家如何使用卡路里计来观测反应中发生的温度变化:Summary
::摘要-
A calorimeter is a device used to measure changes in thermal energy or heat transfer.
::热量计是一种用于测量热能或热传输变化的装置。 -
If a reaction is carried out in the reaction vessel or if a measured mass of heated substance is placed in the water of the calorimeter, the change in the water temperature allows us to calculate the change in thermal energy.
::如果反应容器中进行了反应,或如果将一定量的加热物质放在热量计的水中,水温的变化使我们能够计算热能的变化。
Review
::回顾-
A 300.0 g sample of water at 80.0ºC is mixed with 300.0 g of water at 10.0ºC. Assuming no heat loss to the surroundings, what is the final temperature of the mixture?
::0.0.0°C的A 300.0克样本水与10.0°C的30.0克水混合。 假定周围没有热量损失,该混合物的最后温度是多少? -
A 400.0 g sample of methanol at 16.0ºC is mixed with 400.0 g of water at 85.0ºC. Assuming no heat loss to the surroundings, what is the final temperature of the mixture? The specific heat of methanol is 2450 J/kg•ºC.
::在16.0摄氏度时,甲醇的A400.0克样本与水的400.0克混合,在85.0摄氏度时。假定周围没有热量损失,该混合物的最后温度是多少?甲醇的具体热量为2450焦/千克/摄氏度。 -
A 100.0 g brass block at 100.0ºC is placed in 200.0 g of water at 20.0ºC. The specific heat of brass is 376 J/kg•ºC. Assuming no heat loss to the surroundings, what is the final temperature of the mixture?
::100.0oC时的A100.0g黄铜块在20.0oC时被放在水中20.0g。 黄铜的具体热量为376 J/kg/oC。 假定周围没有热量损失,混合物的最后温度是多少?
Explore More
::探索更多Use this resource to answer the questions that follow.
::使用此资源回答下面的问题 。-
What is the number 4.18 J/g•°C in the video?
::影片中的数字是4.18 J/gC? -
In the equation
, what does
represent?
::在方程式中,C代表什么? -
What does it mean if the temperature in the calorimeter goes down?
::如果卡路里计温度下降是什么意思?
-
A calorimeter is a device used to measure changes in thermal energy or heat transfer.