20.2 原子能
章节大纲
-
You’ve probably used items like those pictured here. What do all of them have in common? They are all used to hold things together. However, they do so in different ways. Like these different kinds of fasteners, there are different kinds of “fasteners” that hold together the subatomic particles inside atoms. The “fasteners” are called forces, and there are three different kinds of them at work inside the : electromagnetic force, strong nuclear force, and weak nuclear force.
::你可能已经使用过像这里所描绘的那些东西。 它们有什么共同之处? 它们都用来把东西放在一起。 但是,它们却用不同的方式把东西放在一起。 和这些不同的紧固装置一样,有各种不同的“快进器 ” , 将原子中的亚原子粒子放在一起。 “快进器”被称为“力量 ” , 而在电磁力、强力核力量和弱力核力量中,它们有三种不同的作用。Electromagnetic Force
::电磁力Electromagnetic force is a force of attraction or repulsion between all electrically charged particles. This force is transferred between charged particles of matter by fundamental force-carrying particles called photons . Because of electromagnetic force, particles with opposite charges attract each other and particles with the same charge repel each other.
::电磁力是所有电荷粒子之间的吸引力或反向力,这种力通过被称为光子的基本载力粒子在有电物质粒子之间转移,由于电磁力,有反电荷的粒子相互吸引,而有相同电荷的粒子相互反射。Inside the atom, two types of subatomic particles have electric charge: electrons, which have an electric charge of -1, and , which have an opposite but equal electric charge of +1. The model of an atom in the Figure shows both types of charged particles. Protons are found inside the nucleus at the center of the atom, and they give the nucleus a positive charge. (There are also in the nucleus, but they have no electric charge.) Negative electrons stay in the area surrounding the positive nucleus because of the electromagnetic force of attraction between them.
::在原子内部,两种亚原子粒子有电荷:电子,其电荷为-1, 电子,其电电荷为+1, 电子电荷为1;原子模型在图中显示两种有电的粒子。质子在原子中心核中发现,它们给予核以正电荷。 (核中也有,但没有电荷。 )负电子留在正核周围,因为它们之间的电磁吸引力。Q: Why do you think protons cluster together in the instead of repelling each other because of their like charges?
::问:你为什么认为质子会聚在一起 而不是因为同样的指控而互相击退呢?A: The electromagnetic force of repulsion between positively charged protons is overcome by a stronger force, called the strong nuclear force.
::A:强力力量,即强力核力量,克服了积极充电质子之间的反弹电磁力量。Strong Nuclear Force
::强力核力量The strong nuclear force is a force of attraction between called , which have a type of charge called color charge. The strong nuclear force is transferred between quarks by fundamental force-carrying particles called gluons. Both protons and neutrons consist of quarks. The exchange of gluons holds quarks together within a proton or neutron. Excess, or residual, strong force holds together protons and neutrons in the nucleus. The strong nuclear force is strong enough to overcome the electromagnetic force of repulsion pushing protons apart. Both forces are represented in the Figure .
::强核力量是被称为“强核力量”之间的一种吸引力量,它具有一种称为彩色电荷的电荷。强核力量通过被称为凝胶的基本载力粒子在夸克之间转移。质子和中子都由夸克组成。质子和中子的交换将夸克放在质子或中子之间。过剩或剩余强力将质子和中子放在核心中。强大的核力量足以克服将质子分离的反向电磁力。两种力量都出现在图中。The types of quarks found in protons and neutrons are called up quarks (u) and down quarks (d). Each proton consists of two up quarks and one down quark (uud), and each neutron consists of one up quark and two down quarks (udd). This diagram represents two protons.
::在质子和中子中发现的夸克类型被称为夸克(u)和下方夸克(d)。每个质子由两个上方的夸克和一个下方的夸克(uud)组成,每个中子由一个上方的夸克和两个下方的夸克(udd)组成。这个图表代表两个质子。The strong nuclear force works only over very short distances. As a result, it isn’t effective if the nucleus gets too big. As more protons are added to the nucleus, the electromagnetic force of repulsion between them gets stronger, while the strong nuclear force of attraction between them gets weaker. This puts an upper limit on the number of protons an atom can have and remain stable. If atoms have more than 83 protons, the electromagnetic repulsion between them is greater than the strong nuclear force of attraction between them. This makes the nucleus unstable, or radioactive, so it breaks down. The following video discusses the strong nuclear force and its role in the atom.
::强大的核力量只在很短的距离内起作用。 结果,如果核核变得太大,它就无效。 随着更多的质子加入核核核心,它们之间的反弹电磁力就会变强,而它们之间的吸引力则会变弱。 这使得一个原子可以拥有并保持稳定的质子数量达到上限。 如果原子有83个质子,它们之间的电磁反弹会大于它们之间的强大核吸引力。 这使得核核或放射性不稳定,因此它就会崩溃。 下面的视频将讨论强大的核力量及其在原子中的作用。Weak Nuclear Force
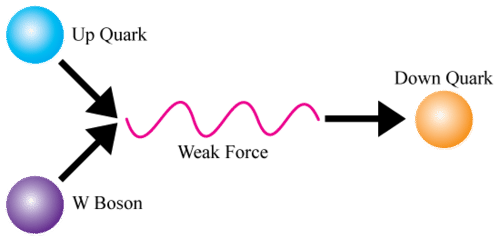
::弱核力量The weak nuclear force is transferred by the exchange of force-carrying fundamental particles called W and Z bosons. This force is also a very short-range force that works only within the nucleus of the atom. It is much weaker than the strong force or electromagnetic force that are also at work inside the atom. Unlike these other two forces, the weak nuclear force does not bind subatomic particles together in an atom. Instead, it changes subatomic particles from one type to another. The Figure shows one way this can happen. In this figure, an up quark in a proton is changed by the weak force to a down quark. This changes the proton (uud) to a neutron (udd).
::微弱的核力量通过武力携带基本粒子(W和Z bosons)的交换而转移。 微弱的核力量也是一种非常短的距离力量, 只在原子核心内起作用。 它比原子内也在起作用的强力或电磁力量弱得多。 与其他两种力量不同, 微弱的核力量不会在一个原子内将亚原子粒子捆绑在一起。 相反, 它会将亚原子粒子从一种类型改变为另一种类型。 图显示一种方式可以发生这种情况。 在这个数字中, 质子的上方方体会被弱力改变为下方。 这把质子( uud) 变成中子( uddd) 。Q: If the weak force causes a proton to change to a neutron, how does this change the atom?
::问题:如果弱力导致质子变成中子,这如何改变原子?A: The resulting atom represents a different . That’s because each element has a unique number of protons. For example, all atoms of helium have two protons. If one of the protons in a helium atom changes to a neutron, the resulting atom would have just one proton, so the atom would no longer be a helium atom. Instead it would be a hydrogen atom, because all hydrogen atoms have a single proton.
::A:产生的原子代表了不同的原子。这是因为每个元素都有独特的质子数。例如,所有原子都有两个质子。如果一个原子中的质子对中子有变化,那么由此产生的原子将只有一个质子,因此原子将不再是原子。相反,它将是一个氢原子,因为所有氢原子都有一个质子。Launch the PLIX Interactive below to build a helium atom. Be sure to use the mass number and atomic number included in the periodic symbol of helium to help you determine the correct number of protons, neutrons and electrons that make up a helium atom:
::启动下面的 PLIX 交互式互动, 以构建 原子 。 请使用 周期符号中包含的质量数和原子数, 帮助您确定组成 原子 的质子、 中子 和 电子 的正确数量 :Summary
::摘要-
The electromagnetic force of attraction between negative electrons and positive protons in the nucleus keeps electrons in the area surrounding the nucleus.
::核中负电子和正质子之间的电磁吸引力 使核心周围的电极保持电子 -
The electromagnetic force of repulsion between positive protons in the nucleus is overcome by the strong nuclear force between protons and neutrons. This force holds the nucleus together.
::质子和中子之间的强力核力量克服了核核核中正质之间的反射电磁力。 -
The weak nuclear force changes subatomic particles from one type to another. When protons change to neutrons, this changes atoms of one element to atoms of a different element.
::弱核力量将亚原子粒子从一种类型改变为另一种类型。当质子变成中子时,这将一个元素的原子改变为另一个元素的原子。
Review
::回顾-
Which subatomic particles are affected by electromagnetic force? How does this force affect them?
::哪个亚原子粒子受到电磁力的影响? -
What is the strong nuclear force? How does it hold the nucleus together?
::什么是强大的核力量?它如何把核核聚在一起? -
How does the weak nuclear force differ from the other fundamental forces inside the atom? How can it change an atom of one element to an atom of a different element?
::弱核力量与原子内其他基本力量有何不同?它如何能将一个元素的原子改变为另一个元素的原子?
-
The electromagnetic force of attraction between negative electrons and positive protons in the nucleus keeps electrons in the area surrounding the nucleus.