5.5 酶和活能能源 -- -- 先进
章节大纲
-
What is the energy needed for biochemical reactions?
::生化反应所需的能量是什么?Is it light or heat? It could be either. But whatever form energy takes, every single in your body - and there are trillions of these reactions (or more) every split second, needs energy to start or activate. And that is known as activation energy .
::它是轻的还是热的?它可以是其中之一。但无论能源的形式是什么,你身体的每一个身体——每分秒就有数万亿个反应(或更多), 需要能量来启动或激活。这被称为激活能量。Activation Energy
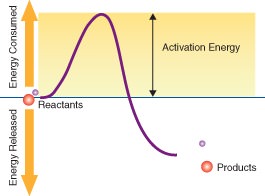
::能源Regardless of whether reactions are exothermic reactions or endothermic reactions , they all need energy to get started. This energy is called activation energy . Activation energy is like the push you need to start moving down a slide. The push gives you enough energy to start moving. Once you start, you keep moving without being pushed again. Activation energy is defined as the energy that must be overcome in order for a chemical reaction to occur, or the minimum energy required to start a chemical reaction. The concept of activation energy is illustrated in Figure .
::无论反应是异热反应还是末温反应,它们都需要能量才能启动。这种能量被称为激活能量。激活能量就像从幻灯片开始向下移动的推力一样。推力使你有足够的能量开始移动。一旦开始,你就可以不再次被推动地继续移动。激活能量被定义为必须克服的能量,以便发生化学反应,或启动化学反应所需的最低能量。激活能量的概念在图中说明。To start this reaction, a certain amount of energy is required, called the activation energy. How much activation energy is required depends on the nature of the reaction and the conditions under which the reaction takes place. Activation energy can be thought of as the height of the energy barrier between the reactants and the products. Why do reactions need energy to get started? In order for reactions to occur, three things must happen, and they all require energy:
::为什么反应需要能量才能开始呢?为了发生反应,必须发生三件事,它们都需要能量:-
Reactant
molecules must collide. To collide, they must move, so they need kinetic energy.
::反应分子必须相撞。要相撞,它们必须移动,所以它们需要动能。 -
Unless reactant molecules are positioned correctly, intermolecular forces may push them apart. To overcome these forces and move together requires more energy.
::除非反应分子的定位正确, 分子的中间力量可能会将它们分开。 要克服这些力量并共同移动,需要更多的能量。 -
If reactant molecules collide and move together, there must be enough energy left for them to react.
::如果反应分子相撞并一起移动, 就必须有足够的能量 让他们作出反应。
Rates of Chemical Reactions
::化学反应率The rates at which chemical reactions take place in organisms are very important. Chemical reactions in organisms are involved in processes ranging from the contraction of to the digestion of food . For example, when you wave goodbye, it requires repeated contractions of muscles in your arm over a period of a couple of seconds. A huge number of reactions must take place in that time, so each reaction cannot take longer than a few milliseconds. If the reactions took much longer, you might not finish waving until sometime next year.
::在生物体中发生化学反应的速度非常重要。 生物体中的化学反应涉及从食物收缩到消化的过程。 例如, 当你挥手告别时, 它需要手臂的肌肉在几秒钟内反复收缩。 大量的反应必须在那个时候发生, 所以每次反应的时间不能超过几毫秒。 如果反应需要更长的时间, 你可能要等到明年才结束挥舞。Factors that help reactant molecules collide and react speed up chemical reactions. These factors include the concentration of reactants and the temperature at which the reactions occur.
::有助于反应分子碰撞和反应加速化学反应的因素,包括反应器的浓度和反应的温度。-
Reactions are usually faster at higher concentrations of reactants. The more reactant molecules there are in a given space, the more likely they are to collide and react.
::反应速度通常较快,反应剂的浓度较高。 特定空间的反应分子越多,它们碰撞和反应的可能性就越大。 -
Reactions are usually faster at higher temperatures. Reactant molecules at higher temperatures have more energy to move, collide, and react.
::高温反应通常更快。 高温反应分子更能移动、碰撞和反应。
Summary
::摘要-
All chemical reactions require activation energy, which is the energy needed to get a reaction started.
::所有化学反应都需要激活能量 这是启动反应所需的能量 -
Rates of chemical reactions depend on factors such as the concentration of reactants and the temperature at which reactions occur. Both factors affect the ability of reactant molecules to react.
::化学反应率取决于反应物浓度和反应发生温度等因素,这两种因素都影响反应分子的反应能力。
Review
::回顾-
What is activation energy?
::什么是激活能量? -
Why do all chemical reactions require activation energy?
::为什么所有化学反应都需要激活能量?
-
Reactant
molecules must collide. To collide, they must move, so they need kinetic energy.