10.22酸雨
章节大纲
-
Why is this gargoyle worn out?
::为什么这个加盖尔磨损了?The construction of Notre Dame Cathedral in Paris, France took nearly 200 years, beginning in 1163 and completing in 1345. Much of the original masonry and decorations, like this gargoyle, were carved from limestone. However, in the 18th and 19th century, increased air pollution in Paris led to acid rain, which caused the stone to much more quickly. Limestone structures around the world are vulnerable to acid rain, which has become more common due to pollutants from fossil fuel burning.
::法国巴黎圣母教堂的建造历时近200年,从1163年开始,到1345年才完工。 原石灰石雕刻了大部分石灰岩和装饰物,如石灰岩,但18世纪和19世纪,巴黎空气污染加剧,导致酸雨,使石块更快地降落。 世界各地的石灰岩结构易受酸雨的影响,酸雨由于化石燃料燃烧产生的污染物而变得更加普遍。Acid Rain
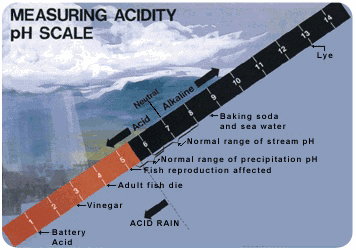
::酸酸雨雨Acid rain is rain that has a pH less than 5 ( Figure ). Acidity is measured on the pH scale . Lower numbers are more acidic, and higher numbers are less acidic (also called more alkaline ). An acid has a pH of less than 7. The pH of normal rain is 5.6. It’s slightly acidic because carbon dioxide in the air dissolves in rain. This forms carbonic acid, a weak acid.
::酸雨是pH值小于5的雨(图 ) 。 酸度在pH值上测量。 低数是酸性的,高数是酸性的(也称为碱性的 ) 。 酸的pH值小于7 。 普通雨的pH值为 5.6 。 因为空气中的二氧化碳会在雨中溶解,所以酸性略高。 这形成了碳酸,一种弱酸。This pH scale includes both normal and acid rain. At what pH do fish have problems reproducing? How Acid Rain Forms
::酸雨形式如何Nitrogen and sulfur oxides are released when fossil fuels are burned. They gases can move downwind and don’t come out of the atmosphere until they dissolve in rainwater ( Figure ). This forms nitric and sulfuric acids. Both are strong acids. Acid rain with a pH as low as 4.0 is now common in many areas. Acid may be even more acidic than acid rain. Fog with a pH as low as 1.7 has been recorded. The acids in are diluted by water , so the damage isn’t immediately noticeable.
::化石燃料燃烧时释放出氮和硫氧化物,气体会向下移动,在溶入雨水之前不会从大气中流出(图 ) 。 这是一种硝酸和硫酸。 这两种酸都是强酸。 酸雨在很多地区都非常常见。 酸可能比酸雨更加酸化。 酸与pH之低记录为1.7。 注入的酸被水冲淡,因此损害不是立即可见的。Nitrogen and sulfur oxides combine with rain to form acid rain. Effects of Acid Rain
::酸雨的影响The image below shows some of the damage done by acid rain ( Figure ). Acid rain ends up in soil and bodies of water. This can make them very acidic. The acid strips soil of its nutrients . These changes can kill trees, fish, and other living things. Acid rain also dissolves limestone and marble. This can damage buildings, monuments, and statues.
::下面的图象显示了酸雨造成的一些损害(图)。酸雨最终会出现在土壤和水体中。这可以使它们变得非常酸化。酸雨将土壤中的养分剥去。这些变化会杀死树木、鱼类和其他生物。酸雨也会溶解石灰岩和大理石。这会破坏建筑物、纪念碑和雕像。Acid rain has killed trees in this forest in the Czech Republic. Summary
::摘要- Nitrogen and sulfur compounds emitted high into the atmosphere create acids. These acids may fall as acid rain.
::高排放到大气中的氮和硫化合物会产生酸,这些酸会随着酸雨而落下。
- Acidity is measured on a pH scale. Rain that is 5.0 or less on that scale is considered acid rain.
::酸度在pH尺度上测量。在该尺度上,5.0或更小的雨被视为酸雨。
- Acid rain weakens plants and animals and damages cultural treasures.
::酸雨会削弱动植物,破坏文化宝藏。
Review
::回顾- Why does acid rain do a lot of damage far from where the smoke was released?
::为什么酸雨会破坏很多 远离烟雾释放的地方?
- What damage does acid rain do to organisms? What damage does it do to cultural structures?
::酸雨会给生物造成什么损害?它会给文化结构造成什么损害?
- Describe the pH scale. What can you say about a liquid with a pH of 2.5? What about a liquid with a pH of 10.5?
::描述 pH 比例尺。 您对pH值为 2.5 的液体有什么看法? 对 pH值为 10.5 的液体有什么看法 ?
Explore More
::探索更多Use the resource below to answer the questions that follow.
::利用以下资源回答以下问题。- What causes acid rain?
::是什么导致酸雨?
- What two gases react with water to make acid rain?
::有两种气体与水产生酸雨反应?
- Where relative to where the gases are emitted does acid rain strike?
::与排放气体的地点相比,酸雨是在哪里发生的?
- What damage can acid rain cause?
::酸雨会造成什么损害?
- How can we reduce acid rain?
::我们如何减少酸雨?
- What are scrubbers?
::什么是洗涤器?
- What is slurry?
::什么是泥浆?
- How effective are scrubbers?
::洗涤器如何有效?
- Nitrogen and sulfur compounds emitted high into the atmosphere create acids. These acids may fall as acid rain.