15.9放射性衰变作为年龄的量度
章节大纲
-
When do you stop counting?
::你什么时候停止计数?Pretend that the large green cylinder is a parent isotope . Now you can visualize the decay of the parent to the daughter. It's easy to see that the second cylinder is half the size of the first. The third is half the size of the second. But when the cylinders get small, the differences are much harder to see. At some point, there is too little of the parent left. That isotope pair is no longer useful for dating.
::假装大绿色气瓶是母同位素。 现在您可以想象女儿的母气瓶衰变。 很容易看到第二个气瓶是第一个气瓶的一半大小。 第三个气瓶是第二个气瓶的一半大小。 但是当气瓶变小时, 差异就很难看出来。 在某些时候, 母气瓶离开的太少了。 这对同位素不再对约会有用 。Radioactive Decay
::放射性衰减Radioactive decay is the breakdown of unstable elements into stable elements. To understand this process, recall that the atoms of all elements contain the particles protons , neutrons , and electrons.
::放射性衰减是不稳定元素分解成稳定元素。 要理解这一过程,请记住所有元素的原子都包含质子、中子和电子。Isotopes
::离线( I)An element is defined by the number of protons it contains. All atoms of a given element contain the same number of protons. The number of neutrons in an element may vary. Atoms of an element with different numbers of neutrons are called isotopes .
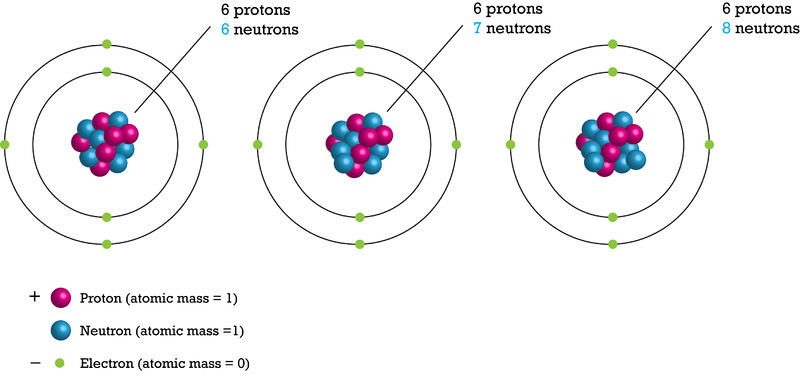
::元素由包含质子的数量来定义。一个元素的所有原子都包含相同的质子数量。元素中的中子数量可能不同。一个元素的原子数量与中子数量不同,其中子数量不同的原子被称为同位素。Consider carbon as an example. Three isotopes of carbon are shown below ( Figure ). All carbon atoms have six protons. But carbon-12 has six neutrons, carbon-13 has seven, and carbon-14 has eight.
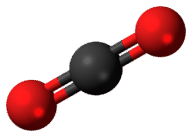
::以碳为例。以下列出了三种碳同位素(图 ) 。 所有碳原子都有6个质子。 但碳-12有6个中子,碳-13有7个,碳-14有8个。Isotopes are named for their number of protons plus neutrons. If a carbon atom had seven neutrons, what would it be named? Almost all carbon atoms are carbon-12, with six protons and six neutrons. Both carbon-12, and the less common carbon-13, are stable isotopes. Only a tiny percentage of carbon atoms are carbon-14, which is an unstable, radioactive isotope of carbon. Carbon-14 forms when cosmic rays form neutrons in the atmosphere . These neutrons collide with nitrogen-14, which is one of the most common gases in the atmosphere. One of nitrogen’s seven protons is replaced with this extra neutron, forming an atom with six protons and eight neutrons. The newly formed carbon-14 atoms in the atmosphere combine with oxygen to form carbon dioxide ( Figure ). Plants take in carbon dioxide during photosynthesis . In this way, carbon-14 enters food chains .
::几乎所有的碳原子都是碳-12,有6个质子和6个中子。碳-12和较不常见的碳-13都是稳定的同位素。只有一小部分碳原子是碳-14,碳-14是一种不稳定的放射性碳同位素。当宇宙射线形成大气中的中子时,碳-14形式是碳-14。这些中子与氮-14相撞,这是大气中最常见的气体之一。氮-14的一个质子被这个额外的中子所取代,形成一个原子,有6个质子和8个中子。大气中新形成的碳-14原子与氧结合形成二氧化碳(Figure )。植物在光合作中吸收二氧化碳。这样,碳-14进入食物链。Carbon-14 forms in the atmosphere. It combines with oxygen and forms carbon dioxide. How does carbon-14 end up in fossils? Decay of Unstable Isotopes
::不稳定伊索托盘衰落Like other unstable isotopes, carbon-14 breaks down, or decays. The original atoms are called the parent isotopes . Eventually, carbon-14 atoms will lose a beta particle, and change to a stable atom of nitrogen-14. The stable atom at the end is the daughter product ( Figure ).
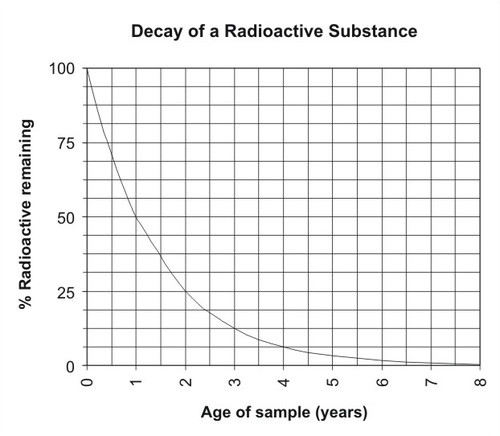
::与其他不稳定的同位素一样,碳-14分解或衰变。原始原子被称为母同位素。最终,碳-14原子将失去一个贝粒,并变成稳定的氮-14原子。最终稳定的原子是母产物(图 )。Unstable isotopes, such as carbon-14, decay by losing atomic particles. They form different, stable elements when they decay. The decay of an unstable isotope to a stable element occurs at a constant rate. This rate is different for each parent-daughter isotope pair. The decay rate is measured in a unit called the half-life . The half-life is the time it takes for half of a given amount of an isotope to decay. For example, the half-life of carbon-14 is 5,730 years. Imagine that you start out with 100 grams of carbon-14. In 5,730 years, half of it decays. This leaves 50 grams of carbon-14. Over the next 5,730 years, half of the remaining amount will decay. Now there are 25 grams of carbon-14. How many grams will there be in another 5,730 years? The figure below graphs the rate of decay of a substance ( Figure ).
::不稳定同位素的衰变到稳定元素的衰变会以恒定速度发生。 每对母-女同位素的衰变速度是不同的。 衰变速度是在一个称为半衰期的单位中测量的。 半衰期是某一同位素的半衰期。 例如, 碳-14的半衰期是5,730年。 想象一下, 你从100克碳-14开始。 在5,730年中, 其中一半衰变。 这留下50克碳-14。 在未来的5, 730年中, 剩余量的一半会衰变。 现在有25克碳-14。 还会有多少克在5, 730年中会衰变? 下图显示一种物质的衰变速度( 图 ) 。The rate of decay of a radioactive substance is constant over time. Summary
::摘要- A half-life is the time it takes for half of the parent isotopes of an element to change to the daughter product.
::半衰期是指将某一元素的一半母同位素转化为女儿产品所需的时间。
- With alpha decay, the nucleus loses two protons and two neutrons.
::随着阿尔法衰变 核会失去两个质子和两个中子
- Carbon-14 has a half-life of 5,730 years.
::碳-14的半衰期为5,730年。
Review
::回顾- What makes an isotope radioactive? Are all isotopes radioactive?
::同位素的放射性是什么?所有同位素都是放射性的吗?
- What is a parent isotope and a daughter product?
::什么是母同位素和女儿产品?
- Describe half-life. Use an example.
::描述半衰期,举一个例子。
- A half-life is the time it takes for half of the parent isotopes of an element to change to the daughter product.