2.2 Stella Spetra
章节大纲
-
In 1835, the French philosopher Auguste Comte predicted that we would never know anything about the chemical composition of stars. He could not have been more wrong. Fraunhofer was already beginning to discover the phenomenon of spectroscopy, and it has turned out to be an extraordinarily powerful technique in astronomy. It has been said (by Harvard astronomer Andrea Dupree in her prize lecture at the AAS meeting in January 2020) that if a picture is worth a thousand words, a spectrum is worth a thousand pictures. With spectroscopy we can trace velocities and we know the composition of stars throughout the galaxy and beyond with exquisite detail. This powerful technique was also used to detect the first planets orbiting other stars.
::1835年,法国哲学家奥古斯特·康特(Auguste Comte)预测,我们永远不会了解恒星的化学构成。他不可能是更错误的。佛劳恩霍费尔(Fraunhofer)已经开始发现光谱学现象,并发现它已成为天文学中极强的技术。据说(哈佛天文学家安德列亚·杜普雷在2020年1月AAS会议上的获奖演讲中说 ) , 如果图片值一千字,那么光谱就值一千幅。光谱可以追溯光谱,我们也能追踪星系和星系以外的星系的速率,并且知道星体的构成。这一强大的技术也被用于探测其他恒星运行的第一颗行星。Spectroscopy
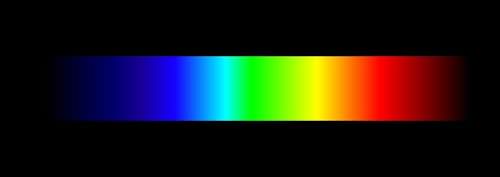
::光谱镜Light is the manifestation of pure energy. It travels at a constant speed in a vacuum. However, there are small wavelength-dependent differences in the speed of light passing through a medium like water or glass. As a result, when white light passes through a prism, it produces a rainbow of color: red, orange, yellow, green, blue, indigo, violet (the notorious ROY G BIV). This apparition of light is called a continuum (or "continuous") spectrum. To the eye, the wavelengths line up as a , uninterrupted sequence. Since our brain interprets different wavelengths of light (in the visible spectrum) as color, we see a familiar, colorful rainbow.
::光是纯能量的表现形式。 它在真空中以恒定的速度运行。 但是, 光通过像水或玻璃这样的介质时, 光的速度有小波长的差别。 因此, 当白光穿过棱镜时, 它会产生彩虹色: 红色、 橙色、 黄色、 绿色、 蓝色、 印地戈、 紫色( 臭名昭著的 ROY G BIV ) 。 这种光的景象被称为一个连续( 或“ 连续” ) 频谱。 对于眼睛来说, 波长会排成一个不间断的序列。 由于我们的大脑将不同的光波长( 可见频谱) 解释为颜色, 我们可以看到一个熟悉的、 彩虹 。The spectrum of white light, dispersed into component wavelengths, produces a rainbow of color. If these wavelengths are individually recombined, what would you see?
::白光的光谱分散在成份波长中,产生彩色彩虹。如果这些波长是个别的重新组合,你会看到什么?When light from the Sun passes through a prism, it casts a rainbow of light as well. However, if the intensity of the rainbow is strong enough and if the dispersion is high enough, narrow dark lines, like those shown in the below, can be observed. Li ght of all wavelengths should be emitted from the Sun - we should see a continuum spectrum. So, why are there dark lines of missing light? What happened to those wavelengths of light?
::当太阳的光穿过棱镜时,它也抛出一道光彩彩虹。然而,如果彩虹的强度足够强,如果弥漫的距离足够高,那么可以观察到狭窄的暗线,如下面所显示的暗线。所有波长的光线都应该从太阳中射出——我们应该看到一个连续的光谱。为什么暗色的光线缺少?这些光的波长发生了什么?When light from the Sun passes through a prism, dark lines can be observed. These features are literally missing light from the Sun. Where did that light go?
::当太阳的光穿过棱镜时,暗线可以观察到。这些特征实际上缺少太阳的光。光去向何方?Let us start the story by tracing the origin of light from the Sun. As we discuss in the next chapter, energy is produced from fusion reactions in the cores of stars. Initially, that energy does not have a continuous spectrum. Most of the radiation from fusion reactions is in the form of high energy gamma rays that human eyes cannot even see. However (as noted in the previous chapter), as those gamma photons leave the core of the Sun, they interact with atoms, or "matter," in the Sun. In some cases, the photons are briefly absorbed by atoms and re-emitted in a random direction. In other cases, the photons simply scatter. Both of these interactions between photons and matter can result in energy loss for the photon.
::让我们从从太阳的光源开始。 正如我们在下一章中讨论的那样, 能量是由恒星核心的聚变反应产生的。 最初, 能量没有连续的频谱。 聚变反应中的大多数辐射是人类眼睛甚至看不到的高能量伽马射线形式。 然而( 如上一章所述 ) , 当伽马光子离开太阳核心时, 它们与太阳原子或“ 物质” 发生互动。 在某些情况下, 光子被原子短暂吸收, 并随机重新释放。 在另一些情况下, 光子只是散射。 光子和物质之间的这些相互作用可能会导致光子的能量损失 。In fact, energy is always conserved during interactions with matter. Energy seems to change because two or more lower energy photons can be emitted in place of the higher energy photon that is absorbed. The sum of the energy from the outgoing photons equals the energy of the incoming photons.
::事实上,能源总是在与物质发生相互作用时得到节能。 能源似乎有所变化,因为可以排放两个或两个以上较低的能源光子来代替吸收的较高能量光子。 离子光子产生的能量总和等于流入光子的能量。[Eqn 1]
::Eclimtii=0nEi+Ei+1...En [Eqn 1]T he energy of a photon is given by (where = Planck's constant and equals the frequency of the photon). The energy of one photon decreases only if one or more additional photons are emitted. This process of going from a fewer number of high energy photons to a larger number of lower energy photons is called thermalization.
::光子的能量由 E=hv (h = Planck的常数,等于光子的频率) 给出 。 光子的能量只有在一个或一个以上额外光子被排放的情况下才会减少。 将少一些高能光子变成更多低能光子的过程被称为热化。The motion of particles undergoing a random walk. This process occurs in the core of the Sun when gamma radiation interacts with atoms and is re-emitted. During these interactions, the photon loses energy and is re-emitted in a random direction. As a result, it takes about 50,000 years (statistically) for energy from the core of the Sun to emerge from the photosphere.
::粒子在随机行走中的运动。 当伽玛辐射与原子发生相互作用并再次传导时, 这一过程会发生在太阳的核心中。 在这些相互作用中, 光子会失去能量, 并在随机方向再次传导。 因此, 从太阳核心中产生能量需要大约50,000年( 统计学上) 才能从光孔中产生能量 。There are billions of interactions between photons and atoms inside the Sun. As a result, a continuum of EM radiation emerges just below the photosphere of the Sun. The photosphere is defined as the layer we "see" - where most of the photons in the photosphere escape and finally begin their journey through space.
::太阳内部有数十亿个光子和原子之间的相互作用。 结果,一连串的电离层辐射在太阳光孔下出现。 光孔被定义为我们“ 看见” 的层, 照片孔中的大部分光子都从那里逃脱, 最终开始穿越太空。We approximate the energy of a star as a blackbody; however, the continuum spectrum is modified as it leaves the photosphere of the star. In this way, a stellar spectrum deviates from a true blackbody spectrum. Because the photosphere is cooler than the inner part of the star, atoms in the photosphere absorb specific wavelengths of light with energies that correspond exactly to the energy required to move an electron from one quantum mechanical state to another.
::我们把恒星的能量比作一个黑体; 但是, 连续波谱随着恒星的光孔而改变。 这样一来, 恒星谱就偏离了真正的黑体谱。 因为光谱比恒星的内部更冷, 光谱中的原子吸收了特定的光波长, 其能量与将电子从一个量子机械状态移动到另一个机械状态所需要的能量完全吻合 。The atom is often diagrammed as a Bohr model with the positively charged protons and neutral neutrons in the nucleus. The number of electrons is equal to the number of protons in an electrically neutral atom. As we will note again when we discuss the chemistry of life, this is a simplification of the fascinating nature of electrons and atoms, but the Bohr model is conceptually useful . In the lowest energy state, the electrons will fill the orbital shells according to the rules of quantum mechanics.
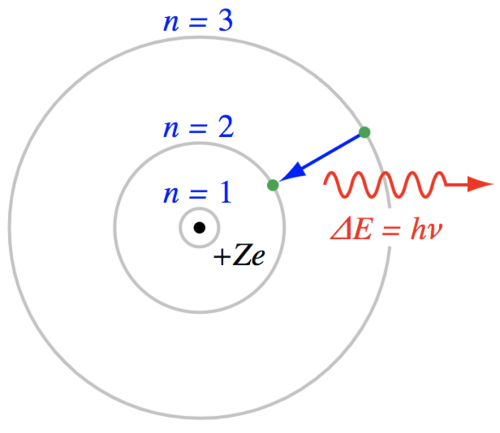
::原子通常被描绘成波尔模型,在核中带有正充电质子和中中中中子。电子数量等于中电子原子中的质子数量。正如我们在讨论生命化学时将再次指出,这是简化电子和原子的迷人性质,但博尔模型在概念上是有用的。在最低能量状态下,电子将根据量子力学规则填充轨道外壳。It seems reasonable that the electrons would not move down to an already filled shell in the atom, but what prevents an electron from moving up to a higher energy shell? It just needs some energy to make that transition. If a passing photon has an energy that is exactly equal to the energy difference between any two orbital shells, there is some statistical probability that the atom will absorb the photon, pushing the electron to a higher energy state. That requirement for photon energy is strict with one exception. Photons with enough energy may completely remove an electron, ionizing the atom and leaving any extra energy in the form of kinetic energy for the atom or electron. But, for generating stellar spectra, the photons of interest have an energy that is matched to the energy difference between one or more orbital shells in the Bohr model.
::似乎合理的是,电子不会下移到原子中已经填满的外壳上,但是什么能阻止电子升到更高的能量壳上?它只需要一些能量才能实现这一转变。如果经过的光子的能量与任何两个轨道外壳之间的能量差异完全相等,那么在统计学上就有一定的概率,那就是原子会吸收光子,将电子推到更高的能量状态。光子能量的要求是严格的,只有一个例外。拥有足够能量的光子可以完全去除电子,使原子电离辐射,并留下任何以动能形式为原子或电子留下的任何额外能量。但是,对于产生恒星光谱,感兴趣的光能与波尔模型中一个或多个轨道外壳之间的能量差异相匹配。Energetically, the process of absorbing a photon is similar to the action of tossing a ball into the air - the ball gains potential energy but then drops back to the ground. L ike the tossed ball, the electron will jump up to a higher orbital shell, but then drop back down to the lower energy state. What happens to the energy when electrons fall back to a lower energy state? The atom loses energy and a photon is emitted . If the electron jumps up one level and drops down to its original level, then the emitted photon has the same energy as the absorbed photon. If the energy of the incoming photon was great enough to push the electron up several energy levels, then a cascade of photons can be emitted as the electron drops through a sequence of orbital shells.
::快速地,吸收光子的过程与将球抛入空气的过程相似 — — 球能增加潜在能量,但随后又跌回地面。像抛球一样,电子会跳到更高的轨道外壳上,然后跌回较低的能量状态。当电子返回低能量状态时,能量会怎样?原子会失去能量和光子被排放出来。如果电子往上跳到一个水平,跌到原来的水平,那么所排放的光子与吸收的光子具有相同的能量。如果即将到来的光子的能量足以把电子推上几个能量水平,那么随着电通过轨道外壳的序列下降,光子的连锁就会被排放出来。Bohr model of the atom is a convenient (though physically incorrect) way to visualize the transition of electrons between energy levels an an atom.
::原子的波尔模型是一种方便(但物理上不正确)的方法,可以想象电子从一个原子的能量水平向一个原子的转换。If the photon is re-emitted, why are there dark lines in the continuum spectrum? When an atom releases the absorbed energy (either as a single photon with the same energy as the absorbed photon or as a cascade of lower energy photons), that energy is emitted in a random direction. The direction of the photon has been changed so that the continuum spectrum is now missing photons at those specific wavelengths of light. T his appears as a dark "absorption" line imposed upon a bright continuum spectrum.
::如果光子再次排放,为什么连续光谱中会有暗线?当原子释放吸收的能量时(或者作为单一光子,与吸收的光子具有同样的能量,或者作为低能量光子的级联),能量会以随机方向释放。光子的方向已经改变,这样连续光谱现在就会在这些特定的波长光中缺少光。这看起来像是一根暗的“吸附线”线,它被强加在亮的连续光谱上。The spectrum from a so-called blackbody in thermal equilibrium is initially a continuum spectrum. If the light from the black body passes through a cooler gas, photons with energy will be absorbed if and only if the photon energy corresponds exactly to the energy spacing between atomic quantum levels. This process superimposes an absorption spectrum on the blackbody (or continuum) spectrum.
::热平衡中所谓的黑体频谱最初是一个连续频谱。如果黑体光线穿过较冷的气体,只有光子能量与原子量水平之间的能量间距完全吻合,光子E=hv才会被吸收。这一过程在黑体(或连续)频谱上添加吸收频谱。There is a third possibility. If electrons are already in an excited state without a background of blackbody energy, say in a heated gas, then the electrons can spontaneously lose energy and cascade down to lower energy levels, releasing photons with energy , where that emitted photon energy is exactly the same as the energy spacing of the atomic quantum levels. This physical process produces an emission spectrum with bright lines at specific wavelengths or energies. These three types of spectra: continuum, absorption, and emission are summarized in the below .
::有第三种可能性。如果电子已经处于一种没有黑体能量背景的兴奋状态,比如在加热气体中,那么电子可以自发地失去能量,向低能量水平递减,释放光子,并释放出能量,而光子能量与原子量水平的能量间距完全相同。这种物理过程产生一个排放频谱,以特定的波长或能量为光线。这三种光谱类型:连续、吸收和排放,见下文。-
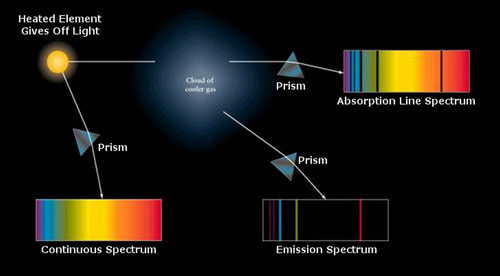
This diagram shows how a light source can produce (1) a continuum spectrum, (2) an absorption spectrum - when light passes through a cooler gas and is absorbed as electrons are pumped up to a higher energy state, or (3) an emission spectrum - when electrons are already in a higher energy state, say in a hot gas, and cascade down, emitting specific wavelengths of light.
::本图显示光源如何产生1) 连续频谱,(2) 吸收频谱----当光穿过冷却气体,当电子被泵到高能量状态时被吸收,或(3) 排放频谱----当电子已经处于高能量状态,例如热气,并逐步下降,释放特定波长的光时。
Most spectra of stars are so-called absorption spectra superimposed on continuum spectra. T he process of absorption is possible because the "blackbody" photons pass through a cooler layer of gas where the electrons are in a lower energy state. This condition is typical for stars. If the photosphere were hotter than deeper layers, then the electrons would already be pumped up to higher energy state and the atoms would not be in a state where they could absorb the emerging photons. The absorption lines in a stellar spectrum represent a deviation from a hypothetical black body, but the blackbody law is still a useful construct for characterizing stellar temperatures.
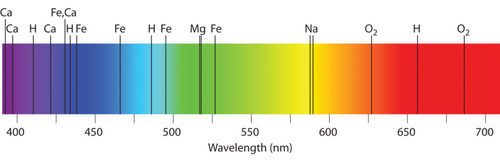
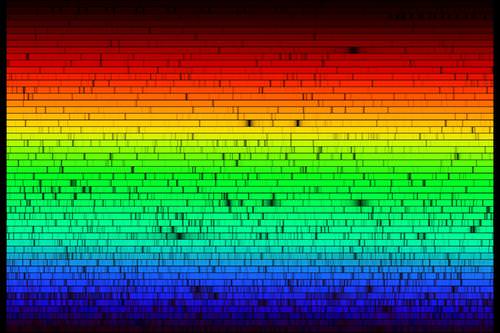
::大多数恒星的光谱都是所谓的吸收光谱,并被叠加到连续光谱中。吸收过程之所以可能,是因为“黑体”光子穿过一个更冷的气体层,因为电子处于较低的能量状态。对于恒星来说,这种情况是典型的。如果光孔比深层更热,那么电子就已经被泵到更高的能量状态,原子就不会处于能够吸收新兴光子的状态。星光谱中的吸收线代表着一种与假设的黑体的偏差,但黑体法则对于确定恒温的特征来说仍然是有用的。Spectral absorption and emission lines were described above as if they were infinitesimally thin lines - exact energies. Quantum mechanics is more complex. Absorption and emission lines are slightly broadened by a number of effects. The Heisenberg uncertainty principle enforces some energy uncertainty in the electron. The rotation of the star will produce Doppler shifts that broaden absorption lines. Gases in the atmosphere of the star may be cooler than the plasma deep in the star, but at temperatures of a few thousand degrees, there is still turbulence, and collisions change the energy of the atoms and therefore the energy of the absorption or emission lines. It is also the case that the ability of a spectrograph to disperse light (the spectral resolution of the instrument) may not be high enough, causing blending of atomic lines. A very high resolution of the Sun is shown below.
::上面将光谱吸收线和排放线描述为微小的细线-精确能量。 量子力学比较复杂。 吸收线和排放线会因一些效应而略有扩大。 海森堡不确定性原则在电子中强制实施某些能源不确定性。 恒星的旋转将产生多普勒变化, 从而扩大吸收线。 恒星大气中的气体可能比恒星深处的等离子体冷, 但是在几千度的温度下, 仍然有动荡, 碰撞会改变原子的能量, 从而改变吸收线或排放线的能量。 光谱仪( 仪器的光谱分辨率) 分散光的能力可能不够高, 导致原子线的混合。 以下显示太阳的高度分辨率。Echelle spectrum of the Sun. Each row is a different wavelength range in the optical spectrum spanning from about 400 nm (blue, bottom) to about 700 nm (red, top). Superimposed on the continuum spectrum are thousands of absorption lines where atoms like hydrogen, sodium, calcium, iron, etc grab light emerging from the core of the star. Some lines are relatively narrow and others are broadened by physical processes in the atmosphere of the star.
::太阳的光谱。 每行的光谱波长范围不同, 从大约400纳米( 蓝色, 底部) 到大约700纳米( 红色, 顶部 ) 。 连续波谱的超强波长范围是千条吸收线, 其中原子如氢、 钠、 钙、 铁等, 从恒星核心产生的抓取光。 有些线相对狭窄, 另一些线则通过恒星大气中的物理过程而扩大。The Doppler effect
::多普勒效果If there is relative motion between the observer and an object like a star, the spectrum of the star will be shifted redward or blueward. This phenomenon is called the Doppler effect. Because the universe is expanding, most galaxies are moving away from us. All stars in the Milky Way galaxy are orbiting around the center of our galaxy. From our perspective, some of these stars appear to be moving toward us and some appear to be moving away from us. In addition, we are making observations of stars from a planet that is revolving around the Sun and spinning about an axis. Everything is moving, and therefore the Doppler effect is extremely important.
::如果观察者与像恒星一样的物体之间有相对运动,恒星的频谱将会向红或向蓝移动。 这种现象被称为多普勒效应。 由于宇宙正在扩大, 大多数星系正在远离我们。 银河系中的所有恒星都在绕着银河系的中心运行。 从我们的角度来看, 有些恒星似乎正在向我们移动, 有些恒星似乎正在向着我们移动。 此外, 我们正在从一个环绕太阳的行星上观测恒星, 并围绕一个轴旋转。 一切都在移动, 因此多普勒效应非常重要 。The basic idea is that light is a type of wave. Try this thought experiment. Imagine that you are on a boat in the ocean bouncing with some frequency because large waves rolling under the boat. If you turn on the motor and point your boat in the same direction that the waves are traveling, the waves will appear to pass by the boat with a lower frequency. It is as if the waves in the ocean stretched out or became longer. Now turn your boat around and travel in the opposite direction, riding into the waves. In this case, the waves bounce the boat with a larger frequency, as if the waves are shorter.
::基本的想法是光是波形的光。 尝试一下这个思考实验。 想象一下, 你正在一艘海上的船上, 因为大波在船下滚动, 以某种频率跳动。 如果您打开马达, 将船指向与波状相同的方向, 海浪似乎会以较低频率从船上经过。 就像海浪延伸或更长。 现在把你的船转过来, 朝相反的方向行驶, 骑进波状。 在这种情况下, 海浪会以更大的频率跳动船只, 就像波状更短一样 。In the thought experiment above, the length of the waves in the ocean did not change. But your relative velocity (the boat on the waves) changed and from your perspective, this led to an "apparent" change in the length of waves. This is the Doppler effect, and the same thing happens when we measure wavelengths of light. If we are heading toward the star (or if the star is heading toward us), then we encounter the photons with a higher frequency. The photons appear to be blue -shifted from our point of view. Conversely, if we are moving away from the star (or the star is moving away from us), we encounter the photons from the star with a lower frequency; the photons appear to be red-shifted.
::在上文的思考实验中,海洋中的波浪长度没有变化。但从你的观点来看,你的相对速度(波浪上的船)变化了,这导致波浪长度的“明显”变化。这是多普勒效应,当我们测量光波长时也会发生同样的情况。如果我们向恒星飞去(或者如果恒星朝我们飞去),那么我们就会以更高的频率看到光子。从我们的角度看,光子看起来是蓝色的。相反,如果我们远离恒星(或者恒星离我们而去),我们就会以较低的频率看到恒星的光;光子看起来是红色的。Note that it is only the "line of sight" that is affected. In the thought experiment, if your boat had been driving parallel to the waves, they would have hit the boat with the same frequency.
::请注意,受影响的只是“视线 ” 。 在思想实验中,如果你的船与海浪平行驾驶,它们会以同样的频率撞上船只。The non-relativistic Doppler equation tells us how the wavelength will change with radial ("line of sight") velocity:
::非相对性的多普勒方程式告诉我们波长会如何随着射线(“视线”)速度变化:[Eqn 2]
::vc [Eqn 2]Equation 2 says that the ratio of the change in wavelength relative to the true wavelength is equal to the ratio of the radial velocity to the speed of light. As an example, imagine that a stellar absorption line at 600 nm is shifted in the observed spectrum to 600.2 nm. Using 3e8 m/s for the speed of light, you can can calculate the relative radial velocity between you and the star to be 100,000 m/s. A good astronomer will figure out their velocity and subtract it to get the velocity of the star. So, if our orbital velocity (a combination of rotation and orbit around the Sun) is 24,000 m/s, then the actual velocity of the star is (100,000 - 24,000) = 76,000 m/s.
::方程式 2 表示波长相对于真实波长的变化比例等于辐射速度与光速之比。 例如, 想象观测到的频谱中600纳米的恒星吸收线被移动到600.2纳米。 使用 3e8 m/s 的光速, 您可以计算出您和恒星之间的相对辐射速度为 100,000 m/s 。 一个好的天文学家将计算出它们的速度, 并减去它, 以获得恒星的速度。 因此, 如果我们的轨道速度( 绕太阳旋转和轨道的结合) 是 24 000 m/s, 那么恒星的实际速度是 ( 100 000 - 24 000) = 76 000 m/s 。Information content of spectra
::光谱的信息内容Stellar spectra reveal many attributes of stars:
::Stellar光谱显示许多恒星的属性:-
Composition: each of those dark absorption lines can be traced to electron transitions in specific atoms. A stellar spectrum is an atomic fingerprint, revealing the elemental composition of the star.
::这些暗吸收线的构成:这些暗吸收线中的每一条都可以追溯到特定原子的电子转换。恒星频谱是原子指纹,显示恒星的元素组成。 -
Temperature: approximating the flux from the star as a black body, we can fit a model to the energy distribution and determine the equilibrium temperature of the stellar photosphere - this is also called the
effective temperature
of the star.
::温度:将恒星的通量与黑体相近, 我们可以将模型与能量分布相匹配, 并确定恒星光孔的平衡温度- 这也称为恒星的有效温度 。 -
Radial velocity: as the star moves along our line of site the absorption lines shift in a predictable way. Every spectral line changes its wavelength by an amount that is proportional to the radial velocity of the star. (Note: we measure only the radial velocity - i.e., the velocity of the star towards us or away from us. This is one dimension even if the velocity of the star has three dimensions.)
::辐射速度 : 当恒星沿着我们的位置移动时, 吸收线会以可预测的方式变化。 每个光谱线的波长变化量与恒星的辐射速度成正比。 (注意: 我们只测量辐射速度 - 即恒星向我们或远离我们的速度。 即使恒星的速度有三个维度, 这也是一个维。 ) -
Surface gravity: high gravity increases the density of atoms in the star and results in more collisions that broaden parts of the absorption lines.
::地表重力:高重力增加恒星原子的密度,导致更多的碰撞,扩大部分吸收线。 -
Rotation speed: as the star spins faster, the absorption lines get broader.
::旋转速度:随着恒星旋转速度加快,吸收线会变得更宽。
Beyond the visible wavelength range
::可见波长范围以外Our human eyes and brains perceive only optical wavelengths of light. But, we can build instruments with detectors that obtain spectra of stars, planets, and the universe in wavelengths beyond the visible band: the infrared, millimeter, and radio wavelengths. Lower energy photons (infrared to radio wavelengths) induce molecular bonds to vibrate or rotate. Like atomic absorption of optical photons, vibrational and rotational energies are quantized. However, these spectra can be complex because many modes of vibration and rotation exist and can couple to other oscillations in the molecule.
::我们的人类眼睛和大脑只能看到光的光波长。 但是,我们可以用探测器来制造仪器,探测到恒星、行星和宇宙的光谱,其波长超出可见波段:红外线、毫米和无线电波长。低能量光(红外线到无线电波长)会诱导分子联结振动或旋转。像光光光光光、振动和旋转能量的原子吸收一样,振动和旋转能量被量化。然而,这些光谱可能很复杂,因为存在许多振动和旋转模式,并且可以与分子中的其他振动模式相组合。The bonds in molecules are weaker than the binding energy of electrons in atoms. Therefore, molecules dissociate in the photospheres of stars like the Sun but can be detected in cooler stars like M dwarfs. Molecules are also ubiquitous in planetary atmospheres. Symmetric molecules (O 2 , H 2 , N 2 ) tend to be spectroscopically inactive in the infrared - they do not have the capacity to absorb infrared photons. But low energy photons can resonate with the bonds of asymmetric molecules (CO 2 , H 2 O, CO), soaking up infrared light.
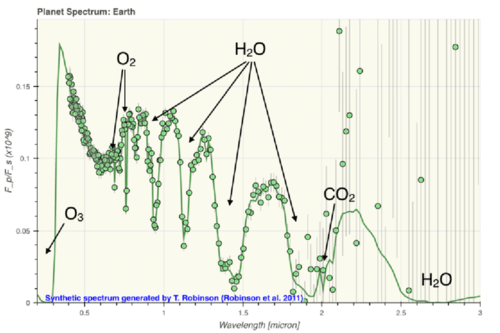
::分子中的联结比原子中电子的紧凑能量要弱。 因此, 分子在太阳等恒星的光谱中分离出来, 但可以在像M侏儒这样的更冷却的恒星中检测到。 分子中的分子也在行星大气中无处不在。 对称分子( O2, H2, N2) 往往在红外线中不活动, 它们没有能力吸收红外线光子。 但是低能量光子可以与不对称分子( CO2, H2O, CO) 的联结产生共鸣, 浸泡红外线光。A synthetic spectrum of the atmosphere of Earth. The oxygen feature appears in visible wavelengths. Asymmetric molecules like H2O, CO2 appear in the infrared.
::地球大气的合成频谱,氧的特征出现在可见波长中,红外线中出现H2O、CO2等非对称分子。The Figure above shows a synthetic spectrum created for a NASA study of future missions to detect Earth-like planets. This simulation is for a relatively low-resolution instrument, so the molecular features appear to be broad and deep. The absorption by symmetrical oxygen atoms can be detected at visible wavelengths, but low energy infrared light excites the vibrational and rotational bonds in asymmetric molecules.
::上图显示了为美国航天局研究未来探测类似地球行星的飞行任务而创建的合成频谱。这种模拟是一种相对低分辨率的仪器,因此分子特征似乎很宽和深。对称氧原子的吸收可以在可见波长中检测到,但低能量红外线光会刺激非对称分子的振动和旋转联系。 -