6.1 前体化学
章节大纲
-
We know that after the Big Bang, there were only a few elements in the Periodic Table: hydrogen, helium, and a sprinkling of lithium. We know that all of the other elements in the Periodic Table were forged during the life and death of stars. We have used spectroscopy to identify the chemical composition of other stars, giant molecular clouds, and even the gas around and between galaxies. The elements in the Periodic Table are ubiquitous.
::我们知道,在大爆炸之后,周期表中只有几个元素:氢、氦和锂的增殖。我们知道周期表中所有其他元素都是在恒星生死期间形成的。我们使用了光谱来辨别其他恒星的化学构成、巨大的分子云,甚至星系周围和星系之间的气体。周期表中的元素无处不在。All of the observations we make of the universe suggest that t he laws governing chemistry and physics in our world also operate in the same way on any other planet at any other location in our galaxy and beyond. Therefore, if we can understand the origin of life on Earth, we would gain insights into when, where, and how life might arise on other worlds .
::我们对宇宙所作的所有观察都表明,我们世界上关于化学和物理学的法律同样适用于银河系内外任何其他地点的任何其他星球。 因此,如果我们能够理解地球上生命的起源,我们就能深入了解其他世界何时、何地和如何出现生命。Chemistry to Biochemistry: CHON(PS)
::生物化学化学:中国(PS)Life is fantastically varied and complex. Yet, all life on Earth is largely composed almost entirely of four elements. Carbon (C), hydrogen (H), oxygen (O), and nitrogen (N) together (CHON) make up approximately 98% of all living things. It is probably not a coincidence that t hese elements are among the most common in the universe (see below). To understand what makes CHON so favorable for life, we first consider the chemistry of these elements .
::然而,地球上的所有生命都几乎全部由四个元素组成。碳(C)、氢(H)、氧(O)和氮(N)合在一起占所有生物的98%左右。这些元素可能是宇宙中最常见的元素(见下文 ) , 可能不是巧合。为了了解什么使中国对生命如此有利,我们首先考虑这些元素的化学作用。
The relative abundances of different elements in the Solar System. What are the most common elements? How do these compare to the elements used by life on Earth?
::太阳系中不同元素的相对丰度。什么是最常见的元素?这些元素与地球上生命所使用的元素相比如何?The chemical reactivity of an element is largely dependent on the arrangement of electrons. Electrons are negatively-charged, sub-atomic particles that surround the positively charged nucleus of an atom. Electrons are organized into quantized energy levels, portrayed as shells in the Bohr model of the atom. The truth about electrons and chemical bonding is far more complex and fascinating than implied by the Bohr model, but we adopt this simplification because it is conceptually clean and sufficient for the discussion here . Q uantum mechanical rules determine the number of electrons that can reside in each electron shell. Electrons in the outermost, or highest-energy level shell of an atom are valence electrons. Valence electrons affect chemical reactivity of an element because this determines the ease and number of chemical bonds that can form.
::元素的化学反应主要取决于电子的安排。 电是负充电的亚原子颗粒,它环绕原子正充电核。 电被组织成量化的能量水平, 在原子的波尔模型中被描绘成贝壳。 电子和化学结合的真相远比博尔模型所隐含的要复杂和迷人得多, 但我们采用这种简化, 因为它在概念上是干净的, 足以在这里进行讨论。 量子机械规则决定每个电子壳中的电子数量。 一个原子的外层或最高能量水平的外层是值电子。 值电子会影响元素的化学反应, 因为这决定了化学联系的易度和数量。The electron structure of the four most common elements in life: (from left to right) hydrogen (H), carbon C), nitrogen (N), and oxygen (O). Large, black circles represent electron shells containing electrons represented by colored dots. Note that actual electron shells do not resemble circles. How many valence electrons does each element have? Do you think that electrons could move up to higher (empty) energy levels? If so, how?
::生命中四种最常见的元素的电子结构是从左到右)氢(H)、碳C(碳C)、氮(N)和氧(O)。大的黑圈代表含有有色点代表的电子的电子贝壳。请注意,实际电子贝壳与圆形并不相似。每个元素有多少价值电子?你是否认为电子能向更高的(空)能量水平移动?如果是,如何呢?
The total number of electrons in an electrically neutral atom is equal to the number of protons (the atomic number). As the above shows, Hydrogen has only one electron while carbon, nitrogen, and oxygen have 6, 7, and 8 electrons respectively. According to the rules of quantum mechanics, the first electron shell can hold two electrons and the second electron shell can hold up to eight electrons. Being electrically neutral is not the lowest energy state for many atoms. The chemistry of elements is driven by the additional stability that occurs when the valence electron shell is filled.
::电子中性原子中的电子总数与质子数量(原子数)相等。 如上所述,氢中子只有1个电子,而碳、氮和氧分别有6、7和8个电子。根据量子力学规则,第一个电子壳可以容纳2个电子,第二个电子壳可以容纳8个电子。电气中性不是许多原子的最低能量状态。元素的化学作用是由等值电子壳填充时产生的额外稳定性驱动的。H ydrogen needs only one electron to complete the first electron shell. If hydrogen simply added an electron, the atom would be negatively charged. Instead, atoms tend to share the electrons that are needed to complete their valence shell. If a shared electron is more tightly held by one atom, then the chemical bond is ionic and the resulting molecule will be polar, with a distortion in the electron cloud that makes one atom slightly more negative and the other atom slightly more positive. If sharing of valence electrons is more equitable , the bond is covalent and the molecule is non-polar. Most chemical bonds in biological organisms are covalent bonds. The below portrays atoms that are sharing their valence shells to aggregate as molecules. Each atom remains neutral, with a completely filled outer electron shell.
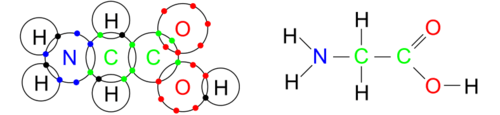
::氢只需要一个电子来完成第一个电子贝壳。 如果氢仅仅添加一个电子,原子就会受到负电荷。 相反,原子往往会分享完成其valence 贝壳所需的电子。 如果共享电子由一个原子更紧密地控制,那么化学联结就是一个离子,由此产生的分子将是极的,电子云的扭曲使一个原子略微负,另一个原子则略微呈阳性。如果共享价值电子更公平,则连接是共价的,分子是非极化的。生物生物生物中的大多数化学联结都是共价联结。下文描述分享其价值贝壳作为分子集成的原子。每个原子都保持中性,有一个完全填满的外电子贝壳。A molecule of glycine, the simplest amino acid. Large, black circles represent the outermost electron shell while colored dots represent electrons. Every hydrogen atom has a full first shell with two electrons while every carbon, nitrogen, and oxygen atom has a full second electron shell with eight electrons. The bonds that are formed are shown to the right as lines between the two elements. Each hydrogen atom has formed one bond while oxygen, nitrogen and carbon have formed 2, 3, and 4 bonds respectively based on their different numbers of valence electrons.
::一种甘油分子, 最简单的氨基酸。 巨大的黑圈代表最外层的电子壳, 而彩色点代表电子。 每个氢原子都有完整的第一颗壳, 配有两个电子, 而每个碳、 氮和氧原子都有完整的第二枚电子壳, 配有八个电子。 所形成的键作为两个元素之间的线向右显示。 每个氢原子组成了一个连接体, 而氧、 氮和碳根据其不同的值电子数量分别组成了2、3和4个链接。Oxygen, with eight electrons, has two electrons in the first electron shell and six valence electrons in the second shell. O xygen needs two additional electrons to fill its outer shell and can form two single or one double bond. Double bonds are shorter and harder to break than single bonds. Because hydrogen and oxygen are so close to a full electron shell, they are chemically aggressive ("highly reactive) in trying to complete their shells. This property makes oxygen very effective in attracting electrons from other atoms.
::氧具有8种电子,在第一个电子壳中有2种电子,在第二个电子壳中有6种等值电子。氧需要另外2种电子填充外壳,并可以形成2个单倍或1倍的双倍保证。双倍保证金比单倍保证金更短、更难打破。由于氢和氧离全电子壳如此近,因此在试图填充外壳时具有化学攻击性(“高度反应性 ”) 。这种特性使得氧在吸引其他原子的电方面非常有效。Nitrogen, with seven electrons, has five valence electrons. This means nitrogen can form up to three bonds: three single bonds, or a single and a double bond, or a triple bond. All thee options are commonly found throughout biochemistry. Oxygen's ability to attract electrons and nitrogen's relatively weak bonding of valence electrons drive many biochemical reactions. Hydrogen, with its one electron, can form bonds wherever shells need to be completed and makes up 59% of elements in life.
::氮有7种电子,有5种价值电子。这意味着氮可以形成3种债券:3种单一债券,或1种单一债券和2种债券,或3种债券。生物化学中通常都能找到所有选择。氧能吸引电子和相对薄弱的氮维值电子联结,这导致许多生物化学反应。氢,用1种电子,可以在需要完成贝壳并占生命元素59%的地方形成债券。Carbon is so central to organic processes that organic chemistry is sometimes defined as the study of compounds that contain carbon. With six total electrons, carbon has four valence electrons occupying its second electron shell, allowing it to form four bonds or a combination of single, double, and triple bonds. Stable bonds form elements like carbon monoxide (CO) or carbon dioxide (CO 2 ). Carbon-carbon bonds in particular are easily made and remain very stable , allowing carbon to form the networked lattice in graphite or diamonds, or long, cyclical structures, or long, complex hydrocarbon molecules.
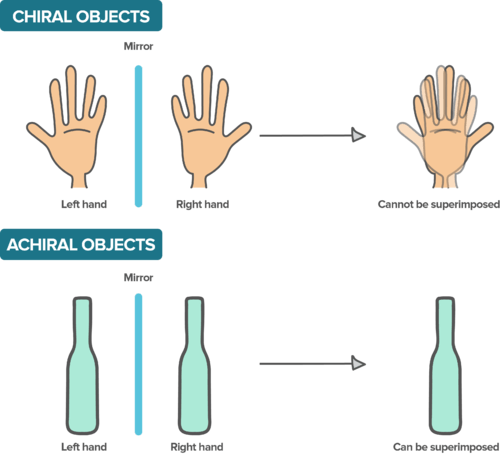
::碳是有机过程的核心,因此有机化学有时被定义为对含有碳的化合物的研究。 碳有6个总电子,有4个价值电子占据其第二个电子壳,允许它组成4个债券或单一、双倍和三倍债券组合。 稳定的债券构成一氧化碳或二氧化碳(CO2)等元素,特别是很容易建立并保持非常稳定,让碳在石墨或钻石或长、周期性结构或长、复杂的碳氢分子中形成网络粘合物。Carbon's ability to form four separate bonds at once allows it to acquire the important property of chirality. Chirality is defined as the property of an object that can not be superimposed on its mirror image. The classic example, illustrated , occurs with your left and right hands. When both hands are palm up, they are mirror images of one another. If you lay your hands flat on a table, it is not possible to slide one hand over the other and match the other hand exactly. For this reason , chirality is often also referred to as "handedness".
::碳能同时形成四个独立的债券, 使它获得重要的天性属性。 偏差被定义为一个对象的属性, 无法在镜像图像上被叠加。 典型的例子, 插图, 出现在你的左手和右手中。 当两只手都掌心的时候, 它们是彼此的镜像。 如果您双手放在一张桌子上, 则不可能将一只手放在另一只手上, 与另一只手完全吻合。 因此, 手性通常也被称为“ 手势 ” 。An example of chiral objects. Hands are chiral objects because their reflections cannot be matched up. Even though this image draws fingers rather poorly, the point is nonetheless made with the thumbs of either hand that are pointing in different directions. A symmetric vase does not have this property because its reflection is no different. Can you think of something that is not symmetric but still achiral?
::手是手性对象的一个例子。 手是手性对象, 因为它们的反射无法匹配。 尽管这张图像画的手指很不好, 但问题还是用指向不同方向的两只手的拇指来表达。 一个对称花瓶没有这个属性, 因为它的反射没有不同。 你能想到一些非对称的, 但仍然神智不清的东西吗 ?Carbon atoms exhibit chirality when they are simultaneously bound to four different things. In such a case, the carbon atom provides a chiral center. Chirality can have a huge impact on the reactivity of a molecule in biological systems. An example is give in the Section below, which discusses chirality in amino acids.
::当碳原子同时与四种不同的东西联系在一起时,碳原子就表现出了手性。 在这种情况下,碳原子提供了一个手性中心。 高度性会对生物系统中分子的回动产生巨大影响。 下面的一节就举了一个例子,其中讨论了氨基酸中的手性。Phosphorus and sulfur, while much less abundant, are nonetheless essential to life as we know it, and warrant an honorable mention with CHON(PS). Phosphorus is involved in forming the borders between cells, the back bone of DNA, and energy storage and transport in cells. Sulfur helps to ensure proteins maintain their shape and carry on important functions.
::磷和硫虽然不那么丰富,但对于我们所知的生命至关重要,值得向中国提及。 磷涉及形成细胞、DNA后骨、能量储存和细胞运输之间的界限。 硫磺有助于确保蛋白质保持其形状和发挥重要功能。Alternative Biochemistries
::替代生物化学While carbon is clearly favored for Earth-based organisms, other hypothetical biochemistries have been considered by biochemists . One of the most frequently imagined alternatives is silicon-based life. Silicon is similar to carbon in that it also has four valence electrons. However, these electrons are in silicon's third electron shell while carbon's valence electrons appear in its second electron shell. The additional shielding of valence electrons by two inner filled shells of electrons is enough to change the nature of silicon bonds. Because of the valence electrons of silicon are farther from the positively charged nucleus, silicon bonds are weaker than carbon bonds . While carbon can effectively form the complex long molecular chains necessary for life, it is rare to find more than three silicon atoms in a single molecule. Even when they do form, compounds with multiple silicon atom have bonds that are easily disrupted by water. The difference in valence shell energy also makes it harder for silicon atoms to form double or triple bonds. Therefore, silicon molecules seldom exhibit chirality.
::虽然碳显然有利于以地球为基础的生物,但生物化学家也考虑了其他假设生物化学。最经常想象到的替代品之一是硅基生命。硅与碳相似,因为它也拥有四种价值电子。然而,这些电子存在于硅的第三个电子罐中,而碳的valence 电子则出现在其第二个电子罐中。由两个内装电子弹壳来额外保护价值电子足以改变硅基。由于硅的值电子离正充电核更远,硅基联结比碳基联结更弱。虽然碳可以有效地形成生命所需的复杂的长分子链,但在单个分子中发现超过三个硅原子是罕见的。即使它们形成时,多硅原子的化合物也很容易受到水的干扰。valence 罐的能量差异也使得硅基电子更难形成双倍或三倍的联结。因此,硅基分子很少展示出气态。Because the valence electrons of silicon are more weakly held to the atom, it is easier for oxygen to bond with silicon, but it is also much harder to break these bonds. Once oxygen has bonded with silicon, it takes a lot of energy to pull it away. Biological processes often require recycling elements into different organic compounds. The difficulty in breaking down silicon-oxygen molecules slows the reaction rates . The structure of silicon-oxygen bonds also renders many important organic molecules unfriendly for life. For instance, we exhale carbon dioxide, CO 2 , as part of the process that generates energy. The silicon counterpart of CO 2 , silicon dioxide, is a solid rather than a gas, making it much harder to dispose of than carbon dioxide. In general, silicon tends to form more solid, crystalline structures when compared to its carbon counterparts.
::由于硅的valence econdene 较弱地掌握在原子上,因此氧较容易与硅结合,但打破这些联系也难得多。氧与硅结合后,需要大量能量才能把它取走。生物过程往往要求将元素回收到不同的有机化合物中。分裂硅氧分子的困难会减缓反应速度。硅氧元素的构造也使得许多重要的有机分子生命不友好。例如,我们排放二氧化碳,二氧化碳,作为产生能源的过程的一部分。二氧化碳的硅对等物是固体的,而不是气体,使得处理二氧化碳的难度大得多。一般而言,硅与碳对等物相比,形成更固体的晶体结构。We should not assume that silicon will "never" be a good choice for biochemistry. However, the laws of chemistry seem to strongly favor carbon-based life, especially in temperature and pressure environments found on Earth. The next time your favorite science-fiction movie portrays silicon-based life, remember that it should expel a mouth full of silicon dioxide crystals with every breath.
::我们不应该认为硅是生物化学的好选择。 然而,化学定律似乎非常偏爱碳基生命,特别是在地球上的温度和压力环境中。 下次你最喜欢的科学幻觉电影描绘硅基生命时,记住它应该把满口硅二氧化水晶的嘴和每一口呼吸都排出出来。Amino Acids to Proteins
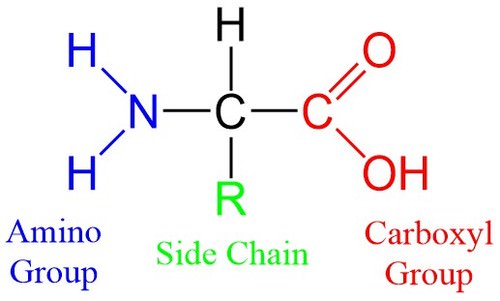
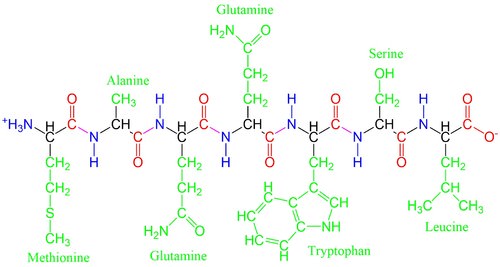
::蛋白质氨酸Amino acids are the building blocks of proteins, and proteins regulate the processes that drive life. Amino acids are defined by a specific, relatively simple structure, and they are found in every living organism on Earth. All amino acids consist of a central carbon atom forming four bonds to: (1) a hydrogen atom, (2) an amino group (-NH 2 ), (3) a carboxyl group (-COOH), and (4) a changeable side chain ( below). In organic chemistry, molecules with a carboxyl group are called carboxylic acids. This along with the amino group gives these compounds the name amino acid.
::氨酸是蛋白质的构件,蛋白质调节着驱动生命的过程。氨酸由特定、相对简单的结构来定义,并且存在于地球上的每个生物体中。所有氨基酸都由中央碳原子组成,组成了四层联结1)氢原子,(2)氨基组(-NH2),(3)氨基组(-NH2),(3)木箱基组(-COOH),(4)可改变的侧链(以下)。在有机化学中,带有碳箱酸组的分子被称为碳箱酸。这与氨基组一起使这些化合物命名为氨基酸。
The components of an amino acid. Each amino acid contains an amino group (blue) and a carboxyl group (red), which gives them the name amino acid. Amino acids acquire different properties by hosting different side chains (green), often denoted by an R or called the R-group. This allows for the wide variety of functionality seen in proteins
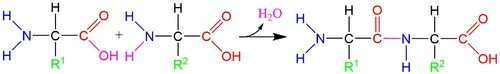
::氨基酸的成分:每种氨基酸都含有一个氨基(蓝)和一个碳盒(红)组(红),它们拥有氨基酸的名称;氨酸通过容纳不同的侧链(绿色)获得不同的特性,通常由R表示,或称为R组,这样就能够在蛋白质中看到各种各样的功能。The amino and carboxyl groups allow amino acids to bond to one another through a process of dehydration with the loss of an -OH group and an -H atom ( below). During this process, a new peptide bond forms between the carbon and nitrogen atoms. Because these molecules are forged through peptide bonds, strings of amino acids attached together are called polypeptide chains. Polypeptide chains are then folded into proteins.
::氨基和碳箱基组允许氨基酸通过脱水过程与一个 -OH组和一个-H原子(以下)的丧失相联。在这一过程中,碳和氮原子之间形成了一种新的基联结形式。由于这些分子是通过基联结制成的,因此连接在一起的氨基酸链被称作聚苯二烯链,然后将聚苯二烯链折叠成蛋白质。The formation of a peptide bond. The -OH from the carboxyl group and the -H from the amino group react to form the peptide bond (purple). These atoms are released as a molecule of water, H 2 O.
::聚氨基氨基氨基磺酰胺(piple)的-OH和-H反应形成聚氨酯联结(piple)。这些原子以水分子H2O的形式释放出来。A polypeptide chain. Pictured above is the chain of the first seven amino acids that make up the OPN1LW gene, which allows humans to distinguish red colors. The different amino acids in the chain feature different side chains, shown and labeled in green. There is no need to memorize the amino acid side chains or their names. Can you identify the repeating pattern of the backbone of the chain?
::聚石化链。 上图是构成 OPN1LW 基因的前七种氨基酸的链条, 它使人类能够区分红色。 不同氨基酸在链条上具有不同的边链, 以绿色显示并贴上标签。 不需要对氨基酸侧链或其名称进行混印。 您能识别链的脊柱重复模式吗 ?The side chains give each amino acid a unique functionality . Amino acids can be positively or negatively charged, water-repellent, bulky, bent into different configurations, etc, depending on their side chains. These differences help the polypeptides fold as they form proteins, bind to specific compounds, or chemically react in different ways.
::边链赋予每个氨基酸一个独特的功能。 氨酸可以有正负充电、水阻塞、体积大、弯曲成不同的配置等,视其侧链而定。 这些差异有助于聚苯醚折叠,因为它们形成蛋白质,与特定化合物结合,或以不同方式发生化学反应。Despite the fact that more than 500 amino acids exist on Earth and despite the great variation that is observed in living organisms, life on Earth uses only 20 different amino acids to form the vast array of proteins. Proteins regulate chemical reactions in all aspects of life. This is similar in spirit to the concept that even though the English languages only uses 26 letters, those same letters have been used to write millions of books.
::尽管地球上存在500多种氨基酸,尽管在活生物体中观察到巨大的差异,但地球上的生命只使用20种不同的氨基酸来形成大量的蛋白质。 蛋白质调节着生命所有方面的化学反应。 这在精神上与这一概念类似,即使英语只使用26个字母,但这些字母也被用于撰写数百万本书。Essential Amino Acids, Body Building, and You
::必需的氨酸, 身体建筑,还有你Of the 20 amino acids used by life on Earth, the human body is capable of synthesizing all but nine. These nine amino acids are known as the essential amino acids. It is important to include sources of these amino acids either from meat or plants in a healthy diet since the body has no other source for them.
::在地球上生命使用的20种氨基酸中,人体能够合成除9种之外的所有物质。这9种氨基酸被称为基本氨基酸,必须将这些来自肉类或植物的氨基酸来源纳入健康的饮食中,因为人体没有其他来源。In fact, many products sell amino acids as supplements targeted towards endurance athletes and body builders. Scientists have been able to trace different amino acids and the role they play in muscle contraction or recovery to identify what the body needs after the coach has said "last set'" for the third set in a row. For example, glutamine is drained during intense physical activity. If the body's glutamine stores become depleted, the body begins to break down muscle cells to compensate.
::事实上,许多产品销售氨基酸,作为针对耐力运动员和身体构造者的补充。 科学家们能够追踪到不同的氨基酸及其在肌肉收缩或恢复中发挥的作用,以确定教练说第三组“最后一组 ” 之后身体需要什么。 比如,在密集的体力活动期间,甲基胺被排干。 如果体内的谷类胺库存枯竭,身体开始破碎肌肉细胞以进行补偿。Prolific Proteins
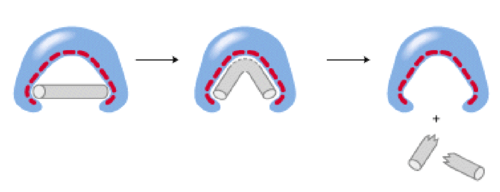
::高蛋白It is commonly said that you are what you eat, but perhaps more correctly , you are what your proteins decide to do. Proteins are the driving force behind the processes of life. Many proteins act as enzymes, which are highly specified molecules that allow complicated organic reactions to progress more easily. In most organic reactions, the molecules involved must first assume an unfavorable, intermediate configuration ( below) before progressing to the finished product. Enzymes bind to these starting molecules and act to stabilize the intermediary state. This makes it easier for molecules to progress to the desired final products. Enzymes are the primary catalyst for organic reactions, and they increase reaction rates for biochemical processes.
::人们常说,你吃什么,但也许更正确,你就是蛋白质决定做什么。蛋白质是生命过程的驱动力。许多蛋白质是酶,这些酶是高度指定的分子,可以让复杂的有机反应更容易地进步。在大多数有机反应中,所涉及的分子必须首先假设一个不利、中间配置(下面),然后才能进入成品。酶与这些起始分子结合,并采取行动稳定中间状态。这使得分子更容易进步到想要的最后产品上。酶是有机反应的主要催化剂,它们提高生物化学过程的反应率。An example of how enzymes operate. If the goal is to bend a metal bar until it breaks, the shape of an enzyme will encourage the bent shape of the metal rather than either the starting bar of the broken pieces. In this example, the bent bar is the transition state that is stabilized. What shape might an enzyme trying to form a musical triangle out of a metal bar look like?
::酶如何运作的一个例子。如果目标是弯曲金属棒直到它破裂,那么酶的形状会鼓励金属的弯曲形状,而不是碎块的起点。在这个例子中,弯曲棒是稳定的过渡状态。试图在金属棒外形成音乐三角形的酶会是什么形状?In addition to acting as enzymes or catalysts, proteins also fulfill several other important roles. Proteins are involved with cell signaling that helps different cells in the body work together. Antibodies are proteins that work with the body's immune system to recognize and destroy foreign substances that might cause illness. Structural proteins give shape or rigidity to cells, such as those that make up our nails or hair. M otor proteins allow for the movement of single-celled organisms. In short, proteins are critical for all of the basic functions of life.
::蛋白质除了作为酶或催化剂外,还发挥其他几个重要作用:蛋白质涉及细胞信号,帮助身体中不同的细胞一起工作;抗体是蛋白质,与身体的免疫系统合作,识别和销毁可能造成疾病的外来物质;结构蛋白质给细胞造成形状或僵硬性,例如构成我们指甲或毛发的细胞;机动蛋白质允许单细胞生物的移动;简言之,蛋白质对于生命的所有基本功能至关重要。Chirality
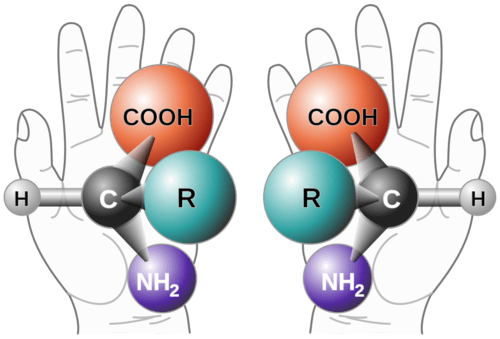
::议会地位The central carbon in an amino acid can serve as a chiral center because it is typically bound to four different groups. The one exception is the amino acid, glycine, whose hydrogen side chain makes it a symmetric molecule . This chirality means there exists both left-handed and right-handed amino acids ( below). Oddly, w hile either configuration is possible, life on Earth only uses left-handed amino acids.
::氨基酸的中央碳可以用作一个手动中心,因为它通常被四组人捆绑在一起。一个例外是氨基酸,甘油,其氢侧链使它成为对称分子。这种手性意味着存在左手和右手氨基酸(下面)。奇怪的是,尽管两种组合都是可能的,但地球上的生命只使用左手氨基酸。The two possible configurations of amino acids with differing chiralities. The R group is pointing out of the page in both cases.
::氨基酸的两种可能配置具有不同的体力,R组在这两处都指出其中的两处。Studies have been done to investigate the functionality of protein chirality. Synthesis of both left-handed and right-handed amino acids is not only possible, i t is chemically equivalent . From an energy standpoint, protein that is composed entirely of right-handed amino acids should function just as well as proteins made of left-handed amino acids. Chirality seems to add an extra layer of regulation in biochemical reactions that may reduce synthesis errors from occurring.
::研究旨在调查蛋白质性能的研究,左手和右手氨基酸的合成不仅有可能,而且具有化学等同性。 从能源角度看,完全由右手氨基酸组成的蛋白质应该与左手氨基酸的蛋白质一样发挥作用。 偏执似乎在生化反应中增加了额外的调控层,这可能会减少合成错误的发生。How did life come to pick left-handed proteins over right-handed ones? There are many competing theories as to the origins of this inequality. One idea is that left-handed amino acids are slightly more water soluble and this could have made them easier to incorporate into early life. Alternatively, light in the protoplanetary disk would have been circularly polarized and it is possible that this might have been more damaging to right-handed amino acids or more favorable to left-handed ones. Amino acids found in space, for example on meteorites, also exhibit an excess of left-handed molecules. If polarized sunlight gave rise to this imbalance it could have tipped the scales to the left for life on Earth. Regardless of what established the original inequality, biological processes probably accentuated the imbalance.
::生命是如何从右手取左手蛋白的呢?关于这种不平等的起源,有许多相互竞争的理论。一个想法是左手氨基酸略微容易溶水,这可以使他们更容易融入早期生活。 或者,原行星盘中的光线会循环地极化,这有可能对右手氨基酸造成更大的损害,或者对左手的氨基酸更为有利。在太空发现的氨基酸,比如在陨石上发现的氨基酸,也表现出了超量的左手分子。如果日光两极化导致这种不平衡,它可能把天体向左边的地球生命倾斜。不管最初的不平等是什么,生物过程可能加重了不平衡。The polarization of light defines how the wave component of light oscillates relative to the direction in which the light is moving. It can be incoherent, as in the bottom right of the figure, oscillate back and forth in only one direction (or linearly polarized as in the center of the figure), or circularly polarized as is shown in the top left of the figure. Different types of filters (shown as blue squares) help give rise to these types of polarization. In the early Solar System, it is thought that dust grains could have caused all light to be circularly polarized.
::光的两极分化决定了光的波成份相对于光向移动方向的振动。 光的波成份可能不连贯, 如在图的右下角, 向一个方向( 或图的中心线性对极化) , 或像图的左上方所示的环极化。 不同类型的过滤器( 以蓝色平方形式显示) 有助于产生这些类型的极化。 在早期的太阳系中, 灰尘粒可能已经导致所有光的环极化 。